- The human immune system is a complex network of specialised cells and organs that protects the body from infections.
- At the core of the immune system is the ability to recognise the difference between self and non-self.
- Autoimmune diseases arise from an overactive and misguided immune response of the body against substances and tissues normally present in the body (self), i.e. the body makes autoantibodies, and actually attacks itself.
- Tissue-specific autoimmune diseases, such as MS (Multiple sclerosis) and RA (Rhematoid arthritis), generally result from a loss of peripheral tolerance to self-antigens, which leads to the inappropriate expansion of the self-reactive effector cell population and causing tissue-specific inflammation.1
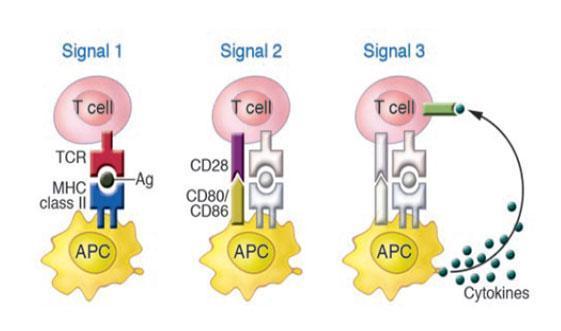
- Antigen presenting cells (APCs) are key players during both the initiation and progression of the autoimmune response.
- APCs perform multifarious tasks in the peripheral immune compartment and, during an immune reaction, provide three signals that are required for the activation of antigen- specific T cells.
- Signal 1 comprises the presentation of antigen peptide, in the context of MHC class II molecules, which is recognized by the antigen-specific TCR.
- Signal 2 involves the stabilization of the synapse through adhesion molecules and the generation of signals via co stimulatory molecules present on the surface of APCs and T cells. CD80/CD86 on APCs interacts with their receptor, CD28, on T cells to generate activatory signals. Thus blocking co-stimulation inhibits T cell activation.
- Signal 3 is produced by the secretion of cytokines by APCs, which signal via cytokine receptors on T cells in order to polarize them toward an effector phenotype as shown in the figure 1. The creation of a particular cytokine environment by APCs during immunity is critical for the determination of the appropriate type of immune response, which can be either cell-mediated or humoral.
- Consistent with this, during an autoimmune reaction, the development of autoreactive T cells into pathogenic and destructive effector cells relies critically upon the secretion of soluble cytokines by APCs. Thus, the capacity of APC-derived cytokines to polarize T cells is of significant importance as it endows APCs with the potential to either promote or suppress the development of autoimmune disease.
Inflammatory cytokines
- Inflammatory cytokines are key regulators of immune responses. But persistent and excessive production of inflammatory cytokines underscores the development of autoimmune diseases.
- Since interferon (IFN) was discovered in 1957, 1,2 more than 90 inflammatory cytokines and their corresponding receptors have been identified 3,4.These cytokines are produced by various cell types, and regulate immune responses, wound healing, angiogenesis, hematopoiesis and tissue remodeling. An appropriate inflammatory response is vital to host defense, whereas excessive or persistent production of inflammatory cytokines results in immunopathology such as inflammatory or autoimmune diseases.
- Autoimmune diseases, such as rheumatoid arthritis (RA) and type 1 diabetes (T1D), have been defined as clinical syndromes that result from inappropriate activation of T cells, B cells or both, such that damage to one or more organ systems occurs 5.
The immune system normally functions to recognise and defend against foreign pathogens by utilising a highly diverse repertoire of specific immune receptors. A large number of these immune receptors recognise self-components, and must be eliminated or silenced by a process known as immune tolerance. Thus develops an ability to differ between self and non-self antigens.
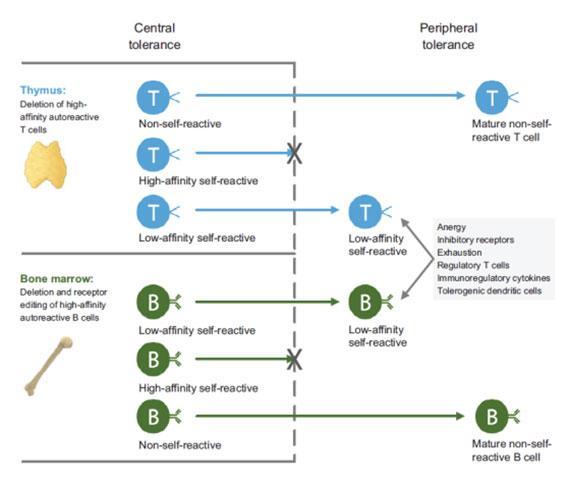
- Central tolerance occurs when high-affinity self-reactive T cells and B cells are eliminated in the thymus and bone marrow, respectively.
- Low-affinity self-reactive T cells and B cells escape central tolerance and enter the periphery, where they are kept in check by complementary and non-redundant peripheral tolerance mechanisms.
- T cell anergy is a tolerance mechanism in which the lymphocyte remains functionally inactive following an antigen encounter, but remains alive for an extended period of time in a hypo responsive state. There may be disruption of the co stimulatory signals or negative interaction with CTLA-4.
- A combination of genetic factors and environmental triggers can lead to disruption of immune tolerance and restoration of the tolerant state is an important goal in the treatment of autoimmune diseases 6.
- In the past 15 years, a number of disease-modifying monoclonal antibodies and genetically engineered biologic agents targeting the immune system have been approved, notably for the treatment of rheumatoid arthritis, inflammatory bowel disease and psoriasis.
Cytokines and Autoimmune diseases
- Upon infection or injury, keratinocytes, macrophages, dendritic cells (DCs), and other cells are activated to produce inflammatory cytokines.
- These inflammatory cytokines in turn act back on macrophages and DCs to induce more inflammatory cytokines, chemokines and other antimicrobial mediators.
- Chemokine recruit myeloid DCs, neutrophils and T cells to the site of infection or injury to further amplify inflammatory responses to protect hosts from infections or to repair tissue damage.
- However, under certain conditions, possibly due to the presence of auto reactive T cells in the case of autoimmunity, there is uncontrolled production of inflammatory cytokines.
- IL-17 and interferon-γ have both inflammatory and homeostatic activities, and, when dysregulated, can promote autoimmunity. In contrast, IL-10 and TGF-β have predominantly regulatory effects in the context of inflammation and might be beneficial in maintaining tolerance and preventing autoimmunity7.
- IL-6 is a key cytokine that has been implicated in autoimmune disease; it supports the development of IL-17-producing T effector cells (Th17) and antagonizes the development of regulatory T cells. This makes IL-6 a potentially powerful target for tolerance-generating therapies8.
Targeting inflammatory cytokines to control Autoimmunity
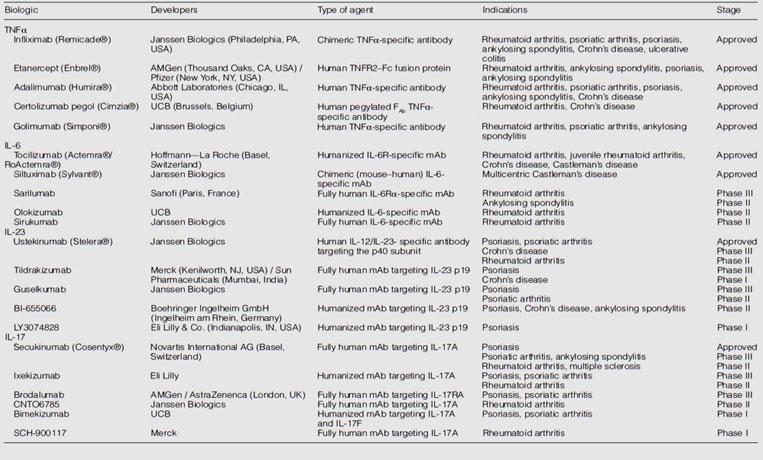
- Although inflammatory cytokines exert variable effects at different stages of different autoimmune diseases, not all of them are effective or promising targets for the treatment of these diseases. Here we outline some of the most effective treatments for autoimmunity. Accumulating pre-clinical and clinical studies show that blocking TNFα, IL-6, IL-23, IL-17 or their corresponding receptors by use of neutralizing antibodies is highly effective in the treatment of multiple autoimmune diseases as they block end organ injury caused by inflammation.
- TNF-axis
- Tumor necrosis factor (TNF-α): TNFα is expressed by several cell types including keratinocytes, macrophages, monocytes, neutrophils, and T lymphocytes while its receptors, TNFR1 (p60) and TNFR2 (p75), are ubiquitously expressed. Deregulated production of TNFα and its receptors is detrimental and has been associated with sepsis and several other inflammatory and autoimmune diseases including RA, psoriasis and colitis.
- Infliximab, etanercept, adalimumab, certolizumab and golimumab are TNF-α blocking antibodies used to treat autoimmune diseases as shown in the table 1.
- Infliximab is an IgG1 mouse–human chimeric antibody and was the first TNFα-targeted drug to be approved, in 1998 9.
- This was followed by the approval of the TNFR2–Fc (crystallizable fragment of immunoglobulins) IgG1 chimera protein etanercept 10.
- Adalimumab was the first fully human antibody targeting TNFα and was approved in 2002. Certolizumab pegol is a new, polyethylene glycol-conjugated, humanized FAb (antigen-binding fragment of immunoglobulins) of a TNFα-specific monoclonal antibody (mAb) and was approved in 2008.
- Golimumab, another fully human antibody, was approved in 2009 11.
- Despite the impressive positive effects of blockade of TNFα in psoriasis, psoriatic arthritis and RA, anti-TNF-α agents worsened disease in patients with MS12 and etanercept failed to treat patients with IBD.
- The IL-23–IL-17 axis
- The pro-inflammatory cytokine IL-23, composed by the two subunits p19 and p40, is mainly produced by inflammatory Dendritic Cells (DCs) within the inflamed skin with the additional contribution of macrophages and keratinocytes. It shares the p40 subunit with IL-12, which contains another subunit—p35. Thus play a key role in driving pathogenic Th17 and γδ T cells to produce high levels of IL-17.13
- IL-23 induces the expansion and the maintenance of the T helper (Th) 17 subsets of T cells. Th17 lymphocytes are characterised by the expression of the transcription factor Retinoic acid receptor-Related Orphan receptor-γt (ROR-γt), typically produce the cytokine IL-17.
- Targeting the IL-23/IL-17 axis has been shown to be a winning strategy in both Psoriasis and Psoriatic Arthritis, as demonstrated by the clinical efficacy of the antagonists currently in use and by the ongoing development of new agents.
- The evidence suggests that targeting the p40 of IL-12 indeed inhibits IL-23 and that inhibiting p40 might be effective to treat psoriasis, too. Therefore, two therapeutic mAbs targeting the p40 subunit, Ustekinumab and briakinumab, were used in treatment of psoriasis (Table 1).
- Both Ustekinumab and briakinumab were effective in Phase III clinical trials for the treatment of psoriasis and psoriatic arthritis4.
- Ustekinumab also showed benefits in the treatment of moderate to severe CD when it was administered as maintenance therapy 14, but failed to prevent inflammation in patients with MS 15.
- Besides Ustekinumab and briakinumab, several IL-23-specific antibodies antagonizing the p19 subunit including tildrakizumab, guselkumab, LY2525623, AMG139, BI-655066 and LY3074828 (Table 1) have been developed 16.
- Tildrakizumab and guselkumab have completed Phase II trials for psoriasis. LY2525623 was terminated in Phase II for complexities in development but not safety concerns. AMG139, BI-655066 and LY3074828 are in early stages of development 16
- Although IL-23 is redundant in host defense to many pathogens, it is important for host responses against Mycobacterium and Candida infections as IL-23-dependent IL-17A and IL-17F play key roles in protecting hosts from these infections 17,18. Therefore, treatment of autoimmune diseases with IL-23 antagonists carries the risk of impaired host defense responses to pathogens. Hence detail molecular and cellular mechanism of anti-IL-23 treatment need to be investigated further.
- IL-17 cytokine
- As a key effector cytokine produced by pathogenic Th17 and γδ T cells, IL-17 plays a crucial role in the pathogenesis of multiple autoimmune diseases such as psoriasis, RA, MS, IBD and myocarditis and has been thought as one of the best targets in the treatment of autoimmune diseases 19.
- There are several monoclonal antibodies targeting IL-17A and IL-17RA have been developed (Table 1).
- The Phase II proof-of-concept studies for secukinumab (a fully human IL-17A-specific monoclonal antibody) , Ixekizumab (a humanized IL-17A-specific antibody) 20 and brodalumab (a fully human IL-17RA-specific monoclonal antibody) 21 showed that each drug was highly effective and helped around 80% of treated patients to achieve a 75% reduction in the Psoriasis Area and Severity Index (PASI).
- Although there are other approaches for treating autoimmune diseases such as cell based therapy, Antigen specific immunotherapy, combination therapy, but all these therapies are not durable and require long term continuation of treatment. Hence Successful restoration of immune tolerance will require innovative approaches utilizing rational targeting of multiple immunological pathways
Quiz
Download the Presentations for this section:
Download these Resources:
References
- Isaacs Jean Lindemann A. Virus Interference: I. The interferon. Proc R Soc London, Ser B, Biol Sci. 1957;147(927):258-267.
- Virus interference. II. Some properties of interferon. Proc R Soc London Ser B – Biol Sci. 1957;147(927):268-273. doi:10.1098/rspb.1957.0049
- Simmons DL. What makes a good anti-inflammatory drug target? Drug Discov Today. 2006;11(5-6):210-219. doi:10.1016/S1359-6446(05)03721-9
- Kopf M, Bachmann MF, Marsland BJ. Averting inflammation by targeting the cytokine environment. Nat Rev Drug Discov. 2010;9(9):703-718. doi:10.1038/nrd2805
- Diseases A. Autoimmune Diseases Resear ch Plan Contents. In: Autoimmune Diseases Resear Ch Plan Contents. ; 2002:83.
- Nepom GT, St. Clair EW, Turka LA. Challenges in the pursuit of immune tolerance. Immunol Rev. 2011;241(1):49-62. doi:10.1111/j.1600-065X.2011.01003.x
- Surh CD, Sprent J. TGF-β puts the brakes on homeostatic proliferation. Nat Immunol. 2012;13(7):628-630. doi:10.1038/ni.2345
- Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235-238. doi:10.1038/nature04753
- R.N. M, F.C. B, J.R. K, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41(9):1552-1563. doi:http://dx.doi.org/10.1002/1529-0131%28199809%2941:9%3C1552::AID-ART5%3E3.0.CO;2-W
- A.A. den B, L.A.B. J, T. S, et al. Long term anti-tumour necrosis factor alpha monotherapy in rheumatoid arthritis: Effect on radiological course and prognostic value of markers of cartilage turnover and endothelial activation. Ann Rheum Dis. 2002;61(4):311-318. doi:http://dx.doi.org/10.1136/ard.61.4.311
- EC K, MC G, L K, et al. – Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly. Ann Rheum Dis. 2009;68:789-796.
- TNF neutralization in MS: Results of a randomized, placebo-controlled multicenter study. Neurology. 1999;53(3):457-457. doi:10.1212/wnl.53.3.457
- Miossec Pierre, Kolls Jay K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763-776. doi:10.1038/nrd3794
- Sandborn WJ, Gasink C, Gao L-L, et al. Ustekinumab induction and maintenance rherapy in refractory Crohn’s disease. N Engl J Med. 2012;367(16):1519-1528. doi:10.1056/NEJMoa1203572
- Segal BM, Constantinescu CS, Raychaudhuri A, Kim L, Fidelus-Gort R, Kasper LH. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7(9):796-804. doi:10.1016/S1474-4422(08)70173-X
- Teng MWL, Bowman EP, McElwee JJ, et al. IL-12 and IL-23 cytokines: From discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21(7):719-729. doi:10.1038/nm.3895
- Puel A, Cypowyj S, Maródi L, Abel L, Picard C, Casanova JL. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol. 2012;12(6):616-622. doi:10.1097/ACI.0b013e328358cc0b
- Khader SA, Pearl JE, Sakamoto K, et al. IL-23 Compensates for the Absence of IL-12p70 and Is Essential for the IL-17 Response during Tuberculosis but Is Dispensable for Protection and Antigen-Specific IFN-γ Responses if IL-12p70 Is Available. J Immunol. 2005;175(2):788-795. doi:10.4049/jimmunol.175.2.788
- Tse MT. IL-17 antibodies gain momentum. Nat Rev Drug Discov. 2013;12(11):815-816. doi:10.1038/nrd4152
- Leonardi C, Matheson R, Zachariae C, et al. Anti–Interleukin-17 Monoclonal Antibody Ixekizumab in Chronic Plaque Psoriasis. N Engl J Med. 2012;366(13):1190-1199. doi:10.1056/nejmoa1109997
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an Anti–Interleukin-17–Receptor Antibody for Psoriasis. N Engl J Med. 2012;366(13):1181-1189. doi:10.1056/nejmoa1109017
References
- Isaacs Jean Lindemann A. Virus Interference: I. The interferon. Proc R Soc London, Ser B, Biol Sci. 1957;147(927):258-267.
- Virus interference. II. Some properties of interferon. Proc R Soc London Ser B – Biol Sci. 1957;147(927):268-273. doi:10.1098/rspb.1957.0049
- Simmons DL. What makes a good anti-inflammatory drug target? Drug Discov Today. 2006;11(5-6):210-219. doi:10.1016/S1359-6446(05)03721-9
- Kopf M, Bachmann MF, Marsland BJ. Averting inflammation by targeting the cytokine environment. Nat Rev Drug Discov. 2010;9(9):703-718. doi:10.1038/nrd2805
- Diseases A. Autoimmune Diseases Resear ch Plan Contents. In: Autoimmune Diseases Resear Ch Plan Contents. ; 2002:83.
- Nepom GT, St. Clair EW, Turka LA. Challenges in the pursuit of immune tolerance. Immunol Rev. 2011;241(1):49-62. doi:10.1111/j.1600-065X.2011.01003.x
- Surh CD, Sprent J. TGF-β puts the brakes on homeostatic proliferation. Nat Immunol. 2012;13(7):628-630. doi:10.1038/ni.2345
- Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235-238. doi:10.1038/nature04753
- R.N. M, F.C. B, J.R. K, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41(9):1552-1563. doi:http://dx.doi.org/10.1002/1529-0131%28199809%2941:9%3C1552::AID-ART5%3E3.0.CO;2-W
- A.A. den B, L.A.B. J, T. S, et al. Long term anti-tumour necrosis factor alpha monotherapy in rheumatoid arthritis: Effect on radiological course and prognostic value of markers of cartilage turnover and endothelial activation. Ann Rheum Dis. 2002;61(4):311-318. doi:http://dx.doi.org/10.1136/ard.61.4.311
- EC K, MC G, L K, et al. – Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly. Ann Rheum Dis. 2009;68:789-796.
- TNF neutralization in MS: Results of a randomized, placebo-controlled multicenter study. Neurology. 1999;53(3):457-457. doi:10.1212/wnl.53.3.457
- Miossec Pierre, Kolls Jay K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763-776. doi:10.1038/nrd3794
- Sandborn WJ, Gasink C, Gao L-L, et al. Ustekinumab induction and maintenance rherapy in refractory Crohn’s disease. N Engl J Med. 2012;367(16):1519-1528. doi:10.1056/NEJMoa1203572
- Segal BM, Constantinescu CS, Raychaudhuri A, Kim L, Fidelus-Gort R, Kasper LH. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7(9):796-804. doi:10.1016/S1474-4422(08)70173-X
- Teng MWL, Bowman EP, McElwee JJ, et al. IL-12 and IL-23 cytokines: From discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21(7):719-729. doi:10.1038/nm.3895
- Puel A, Cypowyj S, Maródi L, Abel L, Picard C, Casanova JL. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol. 2012;12(6):616-622. doi:10.1097/ACI.0b013e328358cc0b
- Khader SA, Pearl JE, Sakamoto K, et al. IL-23 Compensates for the Absence of IL-12p70 and Is Essential for the IL-17 Response during Tuberculosis but Is Dispensable for Protection and Antigen-Specific IFN-γ Responses if IL-12p70 Is Available. J Immunol. 2005;175(2):788-795. doi:10.4049/jimmunol.175.2.788
- Tse MT. IL-17 antibodies gain momentum. Nat Rev Drug Discov. 2013;12(11):815-816. doi:10.1038/nrd4152
- Leonardi C, Matheson R, Zachariae C, et al. Anti–Interleukin-17 Monoclonal Antibody Ixekizumab in Chronic Plaque Psoriasis. N Engl J Med. 2012;366(13):1190-1199. doi:10.1056/nejmoa1109997
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an Anti–Interleukin-17–Receptor Antibody for Psoriasis. N Engl J Med. 2012;366(13):1181-1189. doi:10.1056/nejmoa1109017