Background
- Tolerance is induced and maintained both centrally and peripherally, each with a non-redundant function in maintaining receptor diversity while curtailing self-reactivity
- It was observed more than 50 years ago that non-identical twin calves sharing a placenta do not react to each other’s erythrocytes, and it was hypothesised that in utero exposure rendered them tolerant to each other’s antigens
- This spurred Medawar and Brent to inject mouse cells into a neonatal mouse from a different strain, resulting in tolerance of the latter to skin grafts from the former.
- MacFarlane Burnet pioneered the clonal selection theory: where host encounter with a foreign antigen selects for a particular immune cell clone that then proliferates to yield daughter clones which all have the same specificity
- He hypothesised that self-tolerance resulted from central deletion of ‘forbidden clones’, thereby eliminating potentially self-reactive clones
- Additionally, a deficiency in Treg number or function leads to allergic, autoimmune and other inflammatory diseases due to loss of tolerance
- The most important form of tolerance is self tolerance, which occurs in the developing foetus during normal immune ontogeny
- Tolerance can also be induced to non-self antigens after birth
- An antigen that induces tolerance is termed a tolerogen and tolerance is both antigen- and cell clone-specific: tolerance is selective for the tolerogen that induced it, facilitating continued responsiveness to other antigens
- In this way, tolerance is different from generalised immune suppression (such as that induced by post-transplant drugs like cyclosporine)
Introduction
- The Thymus organ is the seat of T cell development for generating naïve T cells in terms of quantity/number and quality/diversity.
- The thymus is fully developed by the second trimester of embryogenesis, reaches full maturation by age 12 and starts shrinking by adolescence.
- Precursor Hematopoietic Stem Cells (HSC) from the bone marrow, enter the thymus to commit to the T cell linage.
- Thymocytes exit the thymus as mature CD3+ with either CD4 or CD8 single positive T cells
- Very few (<1%) Thymocytes exiting the thymus, may co-express both CD4 and CD8
- Approximately 95% of the mature thymus organ consists of developing thymocytes, with the remaining stroma made up of macrophages, DCs, mesenchymal cells, vascular endothelial cells and thymic epithelial cells (TEC), which orchestrate intra-thymic T cell development.
- Many cytokines (IL7), chemokines (CCR7) and receptors influence the migration, differentiation and proliferation of HSC and thymocytes.
- Various genes, including RAG (recombination activation gene), and transcription factors such as AIRE (autoimmune regulator, linked to promiscuous gene expression) control the development of T cells into subsets, their selection, self-tolerance and regulatory (Treg) capacity.
Central vs. Peripheral Tolerance
- Induction of tolerance requires education of both B and T cells, which occurs in both central (bone marrow, thymus) and peripheral (spleen, lymph nodes) lymphoid organs and tissues
- Here lymphocytes become either immune competent or tolerant towards encountered antigens
- Mechanisms of tolerance induction and maintenance differ between B and T cells, and between central and peripheral lymphoid organs
Central T-Cell Selection
- Transgenic animal models demonstrate that central mechanisms are indispensable for induction of self-tolerance
- CD4-CD8- (double negative) T-cell progenitors enter the thymic cortex and rearrange their receptors to become CD4+CD8+ (double positive) thymocytes
- Positive and negative selection occurs in the thymus (Figure 1)
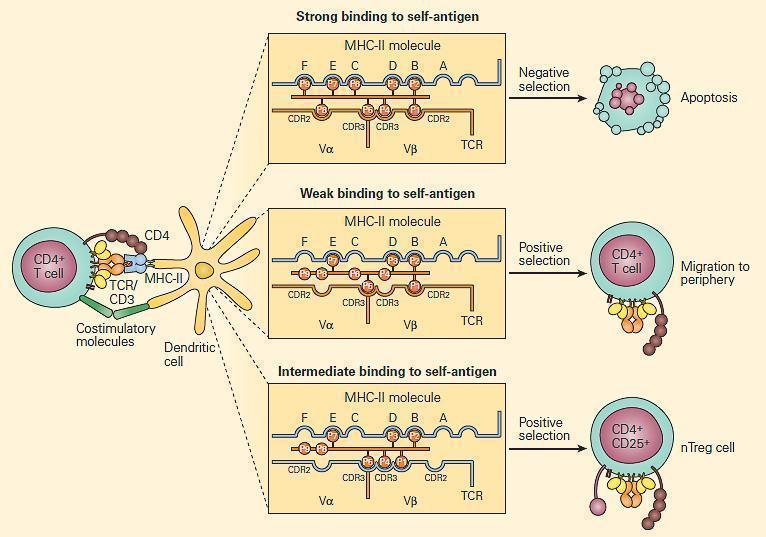
- T-cells with a receptor that bind with moderate affinity to self-peptide-MHC complexes on thymic epithelia receive a survival signal (positive selection)
- Depending on which MHC was recognised, the T-cell will display either CD4 or CD8 (single positive)
- Negative selection occurs at the DP stage in the cortex, or at the SP stage in the medulla: T-cells with a receptor that bind with high avidity to autoantigens on thymic epithelia undergo apoptosis
- The autoantigens are host tissue proteins expressed on thymic epithelia under regulation of the transcription factor autoimmune regulator (AIRE)
- Many T-cells are eliminated: of the potential 109 receptor specificities in the thymus, only a fraction are present in peripheral tissues
- AIRE deficiency results in organ-specific autoimmunity, including APS-1 (damage to parathyroid and adrenal glands)
T cell receptor gene rearrangement
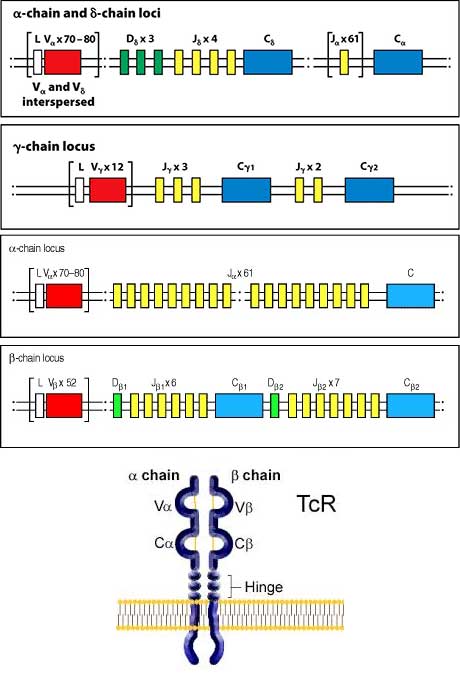
- V(D)JC Gene segments undergo somatic rearrangement to generate diverse TCR repertoire.
- Following rearrangement, P and N nucleotides are also added between the V and J gene segments to achieve virtually infinite diversity.
- Hypervariable regions of the TCR are CDR1, CDR2 and CDR3 (Complementarity determining regions) harbor the highest point of TCR diversity, and molecular characterization of the sequence diversity in this region (spectra typing) is used to determine T cell repertoire.
- T cell repertoire reflects how robust the immune system is able to deal with infectious assault.
- Beta chain rearrangement occurs first to commit HSC to the T cell linage
- Rapid division of thymocytes occurs just preceding and following beta chain rearrangement
- Alpha chain rearranges to pair up with a successfully rearranged beta chain to form alpha beta T cell.
- delta and alpha gene segments are co-located at the same locus (intermixed) on long arm of chromosome 14, therefore rearrangement of delta chain gene segments blocks alpha chain gene segment rearrangement to allow generation of gamma delta T cells.
- The beta and gamma gene segments are located on the short arm of chromosome 7.
Thymic microenvironment and Thymopoeisis
- The thymus consists of 4 compartments (Subcapsule, Cortex, Medulla and cortico-medullary junction)
- The complexity of thymic cellularity reflects the T cell repertoire and facilitates its development.
- TThymocyte development starts with CD4 and CD8 Double Negative (DN) followed by CD4 and CD8 Double Positive (DP) cells, and then CD4 or CD8 Single Positive (SP) mature thymocytes.
- Positive selection occurs within the thymic cortex at the DP stage of thymocyte development.
- Negative selection occurs within the thymic cortex at the DP and SP stages in the medullary compartment.
- Strongly self-MHC-reactive thymocytes are negatively selected in the medulla at a rate of 500,000 per day.
- Limited negative selection reportedly occurs in the thymic cortex, driven by DCs.
- Antigens presented for thymocyte selection arise from the endoplasmic reticulum (ER) compartment but antigen loading may also involve autophagy [“self-eating”, by medullary thymic epithelial cells (mTECs)].
- By interacting with thymocytes, TECs in the cortical (cTEC) and medullary (mTECs) mediate the specific developmental stages of Thymocytes.
- cTECs express and secrete cytokines, chemokine receptor Notch ligand, Delta Like Ligand 4 (DLL4) to drive homing, migration and commitment to the T cell lineage in the DN stage.
- HSC also express chemokine receptors including CCR7 (receptor for CCL19 and CCL21) and CCR9 (receptor for CCL25), enabling HSC colonization of the thymus.
- Throughout the various compartments of the thymus organ, TECs secrete different cytokines to drive migration, induce survival and multiplication of thymocytes prior to maturation and exit via blood vessels, to enter the peripheral circulation as naïve, but fully mature T cells.
- Defects in thymic organ development and cellularity leads to faulty thymopoesis, immune deficiencies and autoimmunity.
- Tregs are generated in the medulla following negative selection and thymocyte maturation, driven by mTECs and DCs.
- IL-7 and FOXN1 are both important for thymus organ development and maintenance including survival and homeostasis.
- APCs (c/pDCs, Macrophages and B cells) are also critical in thymocyte development including selection (positive and negative), tolerance induction and homeostasis, but these are thought to account for less than 1.0% of all APCs in the thymus.
- APCs in the thymus present endogenous and exogenous antigen arising from within the thymus (resident APCs) or imported from the peripheral sites (migratory APCs).
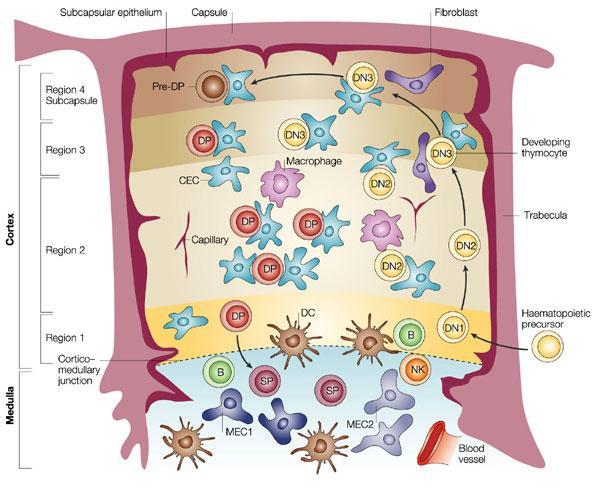
- The different TECs which influence the migration and maturation at the different thymocyte stages through regions 1 to 4 are shown. Bone marrow precursor cells arrive via blood vessels and commitment to the T cell linage is initiated in DN1. In region 2, DN1 differentiates into DN2, proliferate and fully commit to becoming T cells. DN2 cells enter region 3 and differentiate into DN3 and TCR beta (TCRβ) chain gene rearrangement is initiated leading to T cell receptor (TCR) expression on DP cells in region 4. DP cells migrate through the cortex to the medulla; undergo positive selection and become CD4 or CD8 SP cells in the medulla. Mature, but naïve CD4+ and CD8+ T cells then exit the medulla via draining blood vessels into the peripheral circulation.
Central Tolerance generation in the thymus
- Central Tolerance is the ability for T cells to tolerate self-antigens (autoantigens) within the Thymus (Peripheral tolerance generated in spleen and lymphoid tissue).
- Mechanisms of central tolerance in the thymus include:
- Within the cortex, positive selection of thymocytes receiving low intracellular signaling occurs through TCR recognition of self-peptide bound to MHC molecules presented by APCs.
- Negative selection takes place in the medulla, by apoptosis of thymocytes receiving strong intracellular signaling through the TCR, for self-peptide bound to MHC presented by APCs.
- Treg generation in thymic medulla proceeds by anergy of thymocytes receiving intermediate signaling through the TCR, for self-peptide bound to MHC presented by APCs.
- Thymocytes scan TRA (tissue restricted antigen) generated by mTECs to mitigate recognition of all possible epitopes likely to be encountered in the peripheral sites.
- It is possible that rarely occurring mTECs presenting self-antigen in the thymus, that are missed, may lead to autoimmune responses if the T cells in the peripheral sites encounter such lowly represented self-MHC restricted epitopes.
- Promiscuous gene expression by TECs, which is controlled by AIRE, generates diverse self-antigen pool, to selection wide variety of T cell clones forming the T cell repertoire, able to recognize every antigen in the host.
Thymic output and immunosenescence
- The thymus has a limited natural capacity to generate T cells, and suffers age-related decline in function.
- By 18 years of age, thymic decline begins with the onset of involution of the organ.
- Involution is associated with a loss of compartmental architecture and of TEC organization and proportions, and by a decline in naïve T cell production, survival and homeostasis.
- Because the size of the T cell pool remains constant, and without supply of naïve T cells to replenish the pool, expansion of the limited number of T cell clones occurs to fill the gap.
- The accumulation of memory cells, which accompanies homeostatic expansion of fewer T cell clones in old age, results in ‘holes’ in the T cell repertoire, associated with immunosenescence.
- The peripheral T cell pool is affected by a reduced repertoire, proliferation and cytokine production, with increased memory representation, induction of T cell and general lack of immune responsiveness.
- Thymic regeneration including reversal of the effects of thymic involution may be possible with potential to restore naive T cell numbers, variety and immune responsiveness in later life, as well as in infection, as part of immunotherapy measures.
Research tools for Thymus and T cell development studies
- Animal models
- WT/KO/conditional KO mice
- Ultrasound measurements
- Thymus size
- TRECs (T-cell Receptor Excision Circles)
- Thymic output estimation
- Flow cytometry
- Characterization and enumeration of thymocyte populations
- Tracking of thymocyte migration within thymus to better understand developmental niches
- Cell culture
- Thymic organ cultures
- Proliferation assays
- TCRVβ Spectra typing
- T Cell repertoire diversity estimation and monitoring of clonal expansion in disease
- Telomere length estimation
- Determination of T cell and general immunosenescence
Related Talk
Clive Gray, University of Cape Town – Tolerance