Mononuclear Phagocytes Produce Key Inflammatory Cytokines and Chemokines
- Mononuclear phagocytes (monocytes and macrophages) are essential for the development of inflammation and together with neutrophils are the phagocytic cells involved in the clearance of inert particles and microbial agents.
- Monocytes are bone marrow-derived cells and are continuously released into the blood.
- When these cells are recruited by chemotactic molecules and leave the circulation, they become activated and differentiate into macrophages.
- Other names have been applied to tissue macrophages such as Kupffer cells in the liver, histiocytes in connective tissues, mesangial cells in the kidneys, osteoclasts and chondroclasts in bones and cartilage, alveolar macrophages in the lungs, and microglial cells in the brain.
- In contrast to neutrophils, which are end-stage nonreplicating cells continually replaced from the bone marrow, mononuclear phagocytes can proliferate in situ and are long-lived (i.e., months to years).
- Macrophages actually have a number of important functions in body defence such as
- (1) capture by phagocytosis and intracellular killing of microorganisms, infected cells, and tumor cells through PAMP recognition;
- (2) scavenging of worn-out cells, apoptotic bodies, and other debris potentially harmful to tissues;
- (3) processing and presentation of antigens for recognition by T cells, expressing co-stimulatory molecules, mainly during secondary adaptive immune responses;
- (4) releasing cytokines and chemokines that play a major role in innate immune responses.
- (5) regulate immunity through the alternatively activated pathway
- The major cytokines produced by macrophages are TNF-alpha, IL-1 beta, IL-6, and IL-8 and IL-33
- Some important systemic effects of cytokines produced by macrophages are fever induced by IL-1, wasting (i.e., cachexia) caused by TNF-alpha, production of acute phase proteins by liver induced by IL-6, and an increase in the maturation and release of neutrophils from bone marrow by IL-3.
- Although phagocytosis and cytokine/chemokine production are the two key effector functions of macrophages in the innate immune response, these cells also have a role in adaptive responses as antigen-presenting cells and as targets of the effector components of the cellular and humoral adaptive responses, being activated by T cell-derived cytokines and antibodies.
- Macrophage capacity to kill pathogenic microorganisms also can be overcome by microbes that are able to survive inside cells, establishing an intracellular infection, such as Salmonella spp, Mycobacterium tuberculosis, Cryptococcus neoformans, or Toxoplasma gondii.
Dendritic Cells Produce Inflammatory Cytokines and Initiate Adaptive Immunity by Presenting Antigens to T Cells
- Dendritic cells (DCs) are very important innate immune cells that bridge the gap to the adaptive immune system.
- DC are taken to exist in two states: “mature” and “immature”. Maturation is driven by the presence and detection of PAMPs and DAMPs .The main distinguishing of mature DCs is their ability to activate antigen-specific naïve T cells in secondary lymphoid organs. Through this role, DCs are taken to be the most efficient antigen presenting cells for T-cell activation, thereby being the linking the innate and adaptive immune system.
- Based on the molecules they express, cell surface markers, functions and ontogeny, DCs are now taken to fall into the following categories; conventional DCs consisting of two subsets (DC1 and DC2), plasmacytoid DCs (pDC), inflammatory DCs, and Langerhans cells.
- In human blood, the most categories of DCs that are found are:
- (1) pDCs, derived from lymphoid precursors
- (2) cDCs , derived from myeloid precursors
- A distinguishing feature of pDCs is their capacity to produce large amounts of type I interferons, i.e., IFN-α and IFN-β, during the course of viral infections driven by activation via the endosomal Toll-like receptor (TLR)7 and TLR9 pathways
- cDCs respond best to bacterial infection, recognizing lipopolysaccharide (LPS) via the cell-surface TLR4 together with the production of IL-12 and TNF-α.
- Both types of DCs circulate in the blood prior to migration into epithelial sites of skin and mucosal tissues, where they remain as immature DCs.
- These cells utilize the mechanisms of pinocytosis (i.e., ‘‘cell-drinking,’’ a form of endocytosis in which small droplets of liquids are brought into the cell suspended within small vesicles) and phagocytosis for the uptake of a wide variety of foreign proteins and infectious agents.
- This results in the activation of DCs during which the maturation of the cells is promoted with the expression of the chemokine receptor CCR7 allowing the cells to migrate from the periphery to secondary lymphoid organs.
- During emigration, the captured molecules are processed into small peptides, linked to the grooves of MHC-II molecules, and expressed on the cell surface of DCs. By the time DCs reach secondary lymphoid organs, they are able to present antigens to populations of naive and memory T cells.
- Mature, activated DCs can secrete several cytokines and when they interact with CD4+ T-cells, they can influence their polarization into different T helper subtypes based on the particular cytokines present withing the microenvironment, as shown in Figure 1.
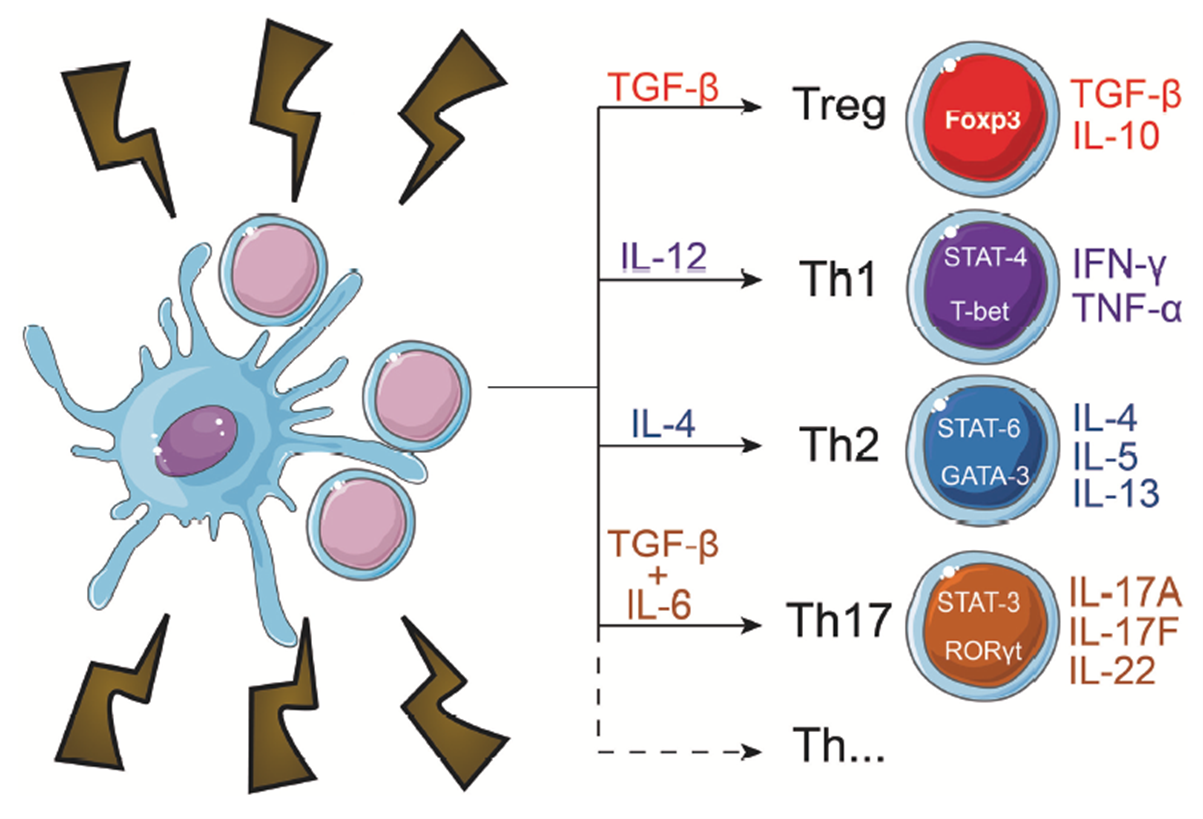
Mast Cell Mediators Regulate a Variety of Physiological Systems
- Mast cells (MCs) originate in bone marrow and circulate as CD34+ progenitor cells, differentiating into mature mast cells under the influence of cytokines only after entry into tissues. Some cytokines taken to play an essential role in both the growth and differentiation of mast cells include SCF, IL-3 and IL-9.
- Mast Cells are distributed throughout the body as resident cells, particularly in association with blood vessels and nerves, and in close proximity to mucosal surfaces that interface with the external environment
- After their development from bone marrow-derived progenitor cells that are primed with stem cell factor (SCF), mast cells continue their maturation and differentiation in peripheral tissue, developing into two well-described subsets of mast cells, MC(T) and MC(TC) on the basis on their enzyme content.
- The first subset consists of MCs containing tryptase (T), i.e., MC(T); the second are mast cells containing both tryptase (T) and chymase (C), i.e., MC(TC).
- The TC type predominates in normal skin and intestinal submucosa, and contains tryptase, chymase, a cathepsin G-like protease, and a carboxypeptidase, whereas the tryptase-containing T type MC(T) is found in intestinal mucosa and lung alveolar wall.
- Mast cells are particularly important in innate immune responses because they are capable of detecting infectious agents and initiating an acute inflammatory response through the secretion of mediators.
- These cells recognize microbial PAMPs through TLR1, -2, -4, and -6 and the complement-derived molecules iC3b, and anaphylatoxins (C4a, C3a, and C5a) through their respective receptors.
- After activation, mast cells immediately extrude granule-stored preformed mediators (heparin, histamine, tryptase, chymase, and carboxypeptidase) and, in minutes, generate lipid-derived prostaglandin D2 (PGD2), and leukotrienes LTC4, LTD4, and LTE4)
- Late, within hours, they synthesize chemokines (CCL3, CCL4, and CCL5) and cytokines (TNF-α, IL-4, IL-5, and IL-6).
- The rapid release of these mediators promotes vascular permeability, induces vasoconstriction, and recruits eosinophils, neutrophils, and other cells within a very short time.
Watch a video of the action of mast cells
Reproduced with permission from Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012.
Basophils and Eosinophils Also Participate in Innate Immunity
- Basophils are granulocytes derived from bone marrow precursor cells and released into the blood circulation.
- Their characteristics and functions are similar to those described for mast cells.
- Eosinophils are another kind of blood granulocyte that can be recruited to sites of innate immune reactions, where their number can be 100 times higher than in the blood.
- Eosinophil cytoplasmic granules contain a variety of cationic proteins that exert several biological effects on normal cells and infectious agents, and these include: major basic protein (MBP), eosinophil cationic protein (ECP), eosinophil-derived neurotoxin (EDN), and eosinophil peroxidase (EPO).
- During the innate immune responses, and induced by phagocytosis of opsonized particles, the eosinophil granule content is released, acting mainly on extracellular helminthic parasites and contributing to tissue damage in inflammatory diseases.
- Other eosinophil products also participate in acute and chronic inflammatory reactions, particularly in allergic diseases (see Chapter 18, Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012).
- These include LT, PAF, and cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-3, IL-5, IL-6, IL-8, TNF-α, and TGF-β.
Natural Killer Cells Recognize and Kill Virus-Infected Cells
- Natural killer (NK) cells are large granular lymphocytes derived from bone marrow precursors and are found mainly in peripheral circulation (5 to 20 percent of total lymphocytes), spleen, liver, and bone marrow.
- Although NK cells belong to the lymphoid lineage, they express neither T (TCR/CD3 complex) nor B (CD19) cell surface markers and are considered the third most important lymphocyte subset (see Chapter 2, Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012).
- NK cells have a morphology similar to that of activated cytotoxic lymphocytes, i.e., a large size, an abundant endoplasmic reticulum (ER), and the presence of preformed granules containing perforins and granzymes.
- Because they express CD16 (Fc-yRIII) and CD56, NK cells are usually identified as CD3-/CD56+/CD16+ cells.
- The role of NK cells in the innate immune response is remarkable because of their nonspecific capacity to eliminate target cells, e.g., virus-infected or malignant cells, through apoptosis independently of T or B cells.
- NK cells also stimulate inflammatory responses through secretion of IFN-gamma, TNF-alpha, GM-CSF, and chemokines (CCL4, CCL5, CCL22) (see Chapter 9, Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012).
- NK cells recognize and kill target cells by apoptosis basically in two ways using an array of different cell surface receptors.
- (1) They utilize the complex balance between activating and inhibitory signals that the NK cell generates to either activate or inhibit their killing activity.
- Some NK cells have receptors generically called killer inhibitory receptors (KIRs) that belong to at least two groups of unrelated molecule structures (C-type lectin and immunoglobulin superfamily), which recognize MHC-I molecules on normal self cells and which, in the normal state, inhibit activation and killing due to the presence in their intracytoplasmatic tail of immunoreceptor tyrosine-based inhibitory motifs (ITIMs) (see Chapter 5, Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012).
- Another diverse set of lectin-type receptors, generically referred to as killer activating receptors (KARs), detect other molecules on altered target cells, e.g., malignant or virus-infected cells, which can provide an activation signal for the NK cell via immunoreceptor tyrosine-based activation motifs (ITAMs).
- If a target cell has decreased expression of MHC-I (see Chapter 20, Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012), the inhibitory signal from KIR will be decreased, shifting the signal balance towards activation of the NK cell and initiating the process of target cell death by apoptosis.
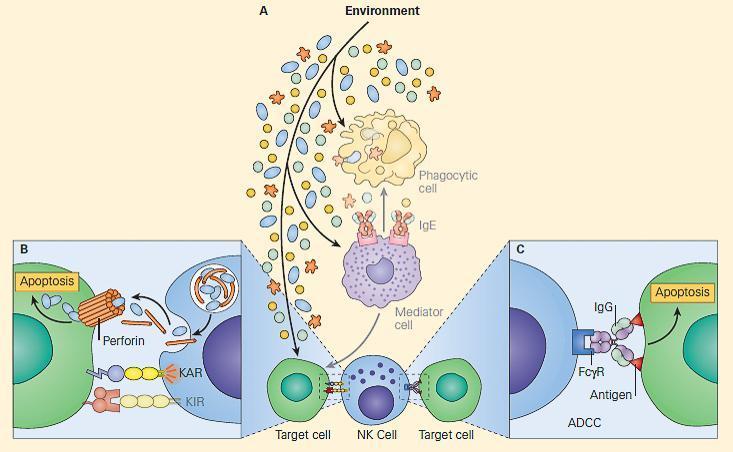
- Once the signal balance is tipped toward activation rather than inhibition, the killing mechanism is initiated, and the NK cells utilize their intracellular granules perforin and granzymes to kill the target cells by inducing apoptosis, shown in Figure 1 (see video below).
- Upon release, perforin in close proximity to a cell slated for killing, first forms pores in the cell membrane of the target cell through which the granzymes and associated molecules then enter, inducing apoptosis.
- 2) The second method whereby NK cells kill target cells is through a hybrid mechanism in which the IgG molecule links the innate and adaptive immune systems and is referred to as antibody-dependent cellular cytotoxicity (ADCC, depicted in Figure 2)
- In this scenario, the NK cell recognizes target cells to which IgG antibody has been attached through its Fab regions.
- At the same time, the Fc portion of the IgG antibody engages the NK cell through its Fc receptor referred to as CD16 (Fc-gRIII), thus linking the NK cell with the target cell.
- Following this linkage, the NK cell can destroy the target cell through induction of the apoptotic pathway.
Natural killer T (NKT) cells
- Another subset of natural killer cells has been identified as NKT cells, which were originally defined by the co-expression of T cell markers (CD3/TCR complex) along with characteristic surface receptors for NK cells (CD56, CD58, and CD161), indicating a dual nature of this subset.
- NKT cells can either express or not express CD4 or CD8 molecules and, similar to NK cells, are considered part of the innate immune response because they act swiftly during infections, killing microorganisms or cells and producing cytokines without the need of slower differentiation or proliferative processes as with T and B cells.
- The original definition of NKT cells, which included a broader group of cells, is now restricted to a more specific subset, the so-called invariant NKT (iNKT) cells.
- They characteristically express on their surface a TCR invariant or constant region (Vα24/J18/Vβ11 in the human) able to recognize glycolipids presented on CD1d molecules
- The CD1 family (CD1a through CD1d) consists of antigen-presenting molecules encoded by genes located outside of the MHC and structurally similar to the MHC-I molecules.
- In comparison to classical MHC molecules, the CD1 antigen-binding groove is highly hydrophobic and adapted for the presentation of lipid antigens that consist largely of glycolipids.
- The cognate α-galactosylceramide (α-Gal Cer, derived from a marine sponge) is the model glycolipid recognized by iNKT cells.
- iNKT cells are present in internal organs, including the thymus, bone marrow, liver, and spleen.
- There are other populations of cells expressing T and NK membrane markers differing from the iTCR cells, but showing variable TCRs, which are able to recognize a broader set of structures in microorganisms, and these are generically called NKT-like cells.
Mucosa-associated invariant T (MAIT) cells
- The term MAIT cells came up as a result of landmark studies that showed how mucosal locations, such as the gut were enriched for these T cells. Further research has gone on to show that within human beings, these cells do have abundant numbers even in non-mucosal tissues, where they account for up to 10% of T-cells in blood.
- They have been placed in the category of ‘unconventional T-cells’ due to the fact that they recognize non-peptide antigens presented by specialized MHC class I–like molecules. The main non-polymorphic MHC class I–like protein that they have been restricted to is called MR1.
- Alongside their MR-1 restriction, all MAIT cells are now taken to be commonly identified by their expression of the invariant TRAV1-2+TRAJ33+ TCR α-chain as well as their ability to bind an MR1–5-OP-RU [5-(2-oxopropylideneamino)-6-D-ribitylaminouracil] tetramer. 5-OP-RU is one metabolite that arises from the riboflavin pathway, a process that occurs in several micro-organisms. Although MAIT cells can respond to several pathogens, they have been noted to have a specificity for antigens from highly conserved bacterial and fungal riboflavin-synthesis pathways.
- Initially taken to be double negative for CD4- and CD8-, MAIT cells can now be put into 5 categories based on the difference in expression of their co-receptors. These subsets include CD4+CD8–, CD4+CD8+, CD4–CD8–, CD4–CD8αα+ and CD4–CD8αβ+ (Gherardin et al, 2018)
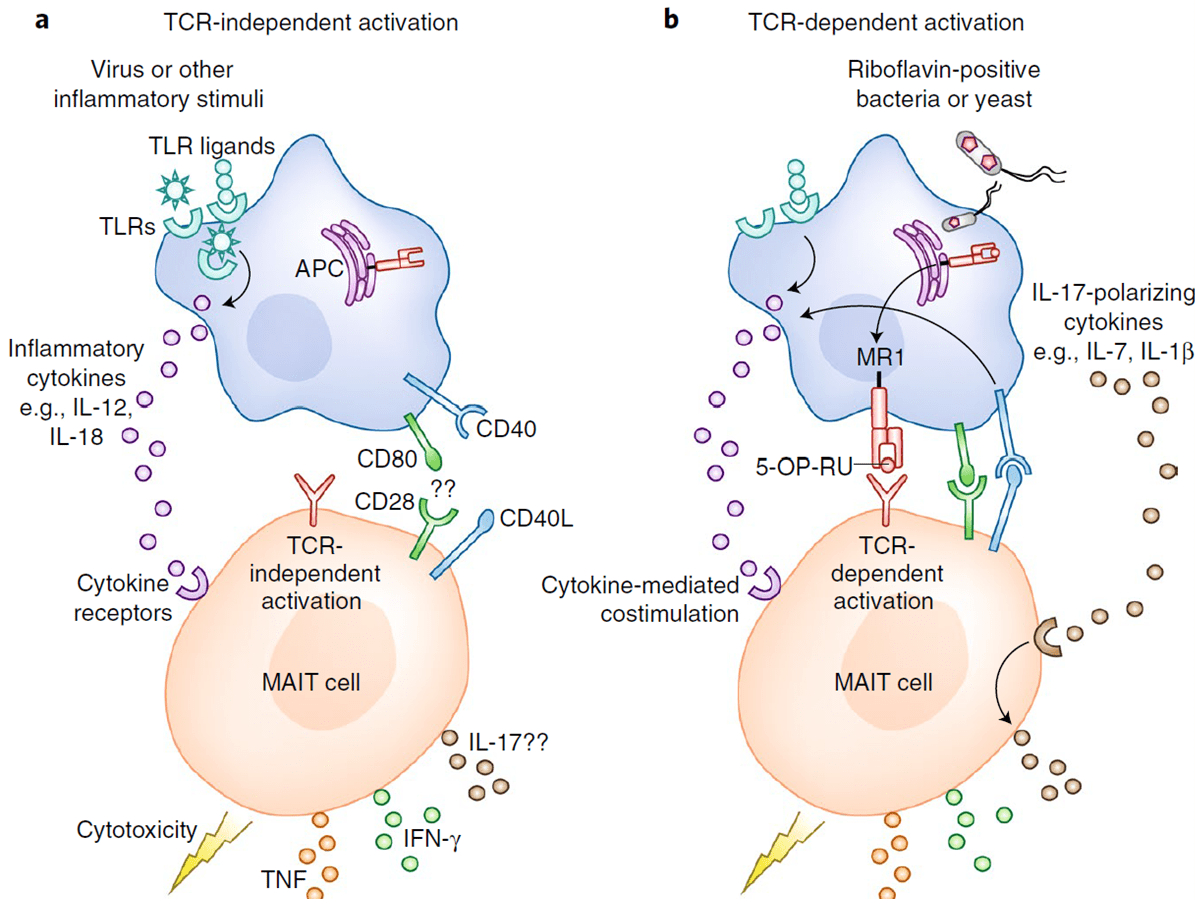
- In spite of the expression of a TCR, recent developments have shown that MAIT cells can be activated with or without the need for TCR engagement, as shown in Figure 2. The presence of at least two or more cytokines such as IL-12 and IL-18 has been noted to drive the production of IFN-γ, TNF and granzyme B by MAIT cells, in a TCR-independent manner (Godfrey et al, 2019).
- Studies carried out mainly in animal models do support the hypothesis of MAIT cells offering protection in most disease states. One example would be where impaired T-cell responses were noted during the early stages of infection with Mycobacterium bovis BCG in MR-1 deficient mice. Polymorphisms in the MR1 gene in human beings have now gone on to be associated with increased susceptibility to TB disease (Seshadri et al, 2017), indicating that there is a role they play when it comes to the immune response to mycobacterium tuberculosis.
B-1 cells
- B-1 cells are a taken to be phenotypically and functionally distinct from conventional B-cells, also known as B-2 cells
- B-1 cells are taken to self-renewing, since they give rise to more mature naive cells by dividing in peripheral lymphoid tissues. Conventional B-2 cells, on the other hand divide only in response to antigen and give rise to memory or plasma cells in the periphery.
- B-1 cells are known to spontaneously secrete ‘‘natural’’ IgM antibodies in the absence of any apparent stimulation by specific antigens (see Chapter 2) for example those stemming from immunisation.
- B-1 cells experience very limited somatic hypermutation. They are taken to be polyreactive as a result of them responding to antigens originating from different pathogens, and binding to these various antigens with very little affinity.
- Accordingly with the expression of CD5 molecules, B-1 cells are subdivided into B-1a that carry the CD5 molecule and B-1b that do not
- B-1a cells represent the majority of B-lineage cells during neonatal life but decline thereafter.
- The repertoire of natural antibodies is much more restricted than those produced by conventional B cells and a large proportion are poly-reactive, meaning that they canresponding to antigens originating from different pathogens, and binding to these various antigens with very little affinity. Examples of these antigens include phylogenetically conserved structures such as nucleic acids, heat shock proteins, carbohydrates, and phospholipids.
- The antibodies produced by B-1 cells may participate as a bridge between innate and adaptive immunity and make an optimal transition between the two immune responses by producing the first wave of antibodies required for antigenic clearance of viruses, bacteria, and certain parasites.
- Other functions performed by these cells are in the immune regulation through the synthesis of IL-10 and in the clearance of senescent and apoptotic cells.
- It has been suggested that they are also involved in autoimmunity as increases in CD5+ B cell frequency have been reported in patients suffering from rheumatoid arthritis, Sjögren’s syndrome, myasthenia gravis, insulin-dependent diabetes mellitus, and Hashimoto’s thyroiditis.
Marginal zone B (MZ B) cells
- MZ B cells are a rare non-recirculating subset of mature peripheral B cells exclusively located in the spleen, different from the more abundant recirculating follicular B cell subset.
- The MZ B cell population is separated from the B cell follicle by the marginal sinus.
- MZ B cells express high levels of CD1d and CD21 molecules.
- They are quick and easily activated by low levels of antigen, are potent antigen-presenting cells for naive T cells, and produce short-lived IgM antibody-forming cells involved in the early defense against blood-borne pathogens, as well as in autoreactive B cell responses.
Watch a video of the different killing mechanisms, including NK cells and ADCC
Reproduced with permission from Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012.
Faculty Presentations
Quiz
References
- Gherardin, N. A. et al. Human blood MAIT cell subsets defined using MR1 tetramers. Immunol. Cell Biol. 96, 507–525 (2018).
- Godfrey, D.I., Koay, HF., McCluskey, J. et al. The biology and functional importance of MAIT cells. Nat Immunol 20, 1110–1128 (2019). https://doi.org/10.1038/s41590-019-0444-8
- Patente TA, Pinho MP, Oliveira AA, Evangelista GCM, Bergami-Santos PC and Barbuto JAM (2019) Human Dendritic Cells: Their Heterogeneity and Clinical Application Potential in Cancer Immunotherapy. Front. Immunol. 9:3176. doi: 10.3389/fimmu.2018.03176
- Seshadri, C. et al. A polymorphism in human MR1 is associated with mRNA expression and susceptibility to tuberculosis. Genes Immun. 18, 8–14 (2017).
References
- Gherardin, N. A. et al. Human blood MAIT cell subsets defined using MR1 tetramers. Immunol. Cell Biol. 96, 507–525 (2018).
- Godfrey, D.I., Koay, HF., McCluskey, J. et al. The biology and functional importance of MAIT cells. Nat Immunol 20, 1110–1128 (2019). https://doi.org/10.1038/s41590-019-0444-8
- Patente TA, Pinho MP, Oliveira AA, Evangelista GCM, Bergami-Santos PC and Barbuto JAM (2019) Human Dendritic Cells: Their Heterogeneity and Clinical Application Potential in Cancer Immunotherapy. Front. Immunol. 9:3176. doi: 10.3389/fimmu.2018.03176
- Seshadri, C. et al. A polymorphism in human MR1 is associated with mRNA expression and susceptibility to tuberculosis. Genes Immun. 18, 8–14 (2017).