What are MDSCs?
- Myeloid-derived suppressor cells (MDSCs) are a group of myeloid cells with potent immunoregulatory activity.
- Two major MDSC subsets have been identified in humans: granulocytic or polymorphonuclear MDSCs (PMN-MDSCs) and monocytic MDSCs (M-MDSCs):
- PMN-MDSCs are phenotypically and morphologically similar to neutrophils. They are defined as CD11b+CD14–CD15+ (or CD66b+)CD33+.
- M-MDSCs are similar to monocytes. They are defined as CD11b+CD14+CD15–CD33+HLA-DR–/lo.
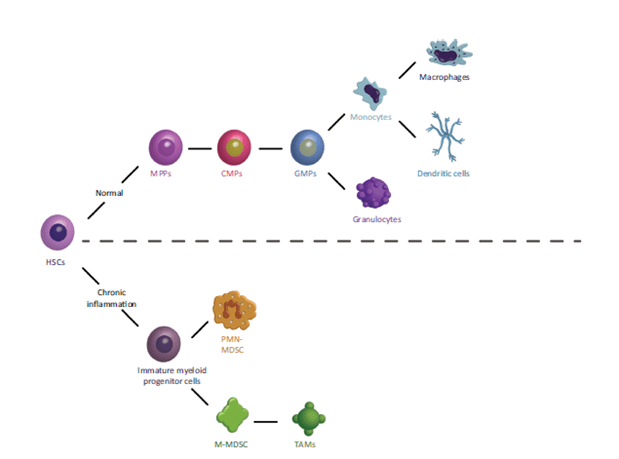
Origin and nature of MDSCs
- Healthy people do not have MDSCs, because the normal process of myelopoiesis consists of immature myeloid cells (IMCs) that differentiate into mature granulocytes, macrophages or dendritic cells. Under pathological conditions like infection, trauma and cancer, inflammatory cytokines such as soluble tumor necrosis factor (sTNF) cause proliferation and differentiation of myeloid cell precursors into MDSCs (Figure 1).
- G-CSF and M-CSF induce the differentiation of granulocytes and macrophages, respectively. In cancer and other pathological conditions, these factors are overproduced and favor the generation of MDSCs.
Function of MDSCs
MDSCs mediate immune suppression through several mechanisms:
- The most prominent factors implicated in MDSC suppressive activity include arginase 1 (ARG1), nitric oxide (NO), up-regulation of reactive oxygen species (ROS), and production of prostaglandin E2 (PGE2).
- MDSCs promote conversion of naive CD4+ T cells into induced regulatory T (iTreg) cells via mechanisms not completely understood, but that may involve cell-to cell contact, including CD40–CD40L interactions, production of soluble factors by MDSCs, such as IFNγ, IL-10, and TGF-β1, and possibly also their MDSC expression of ARG.
- MDSCs inhibit the proliferation of CD4+ T cells via L-arginine depletion by ARG1-dependent consumption.
- MDSCs modulate the cytokine production of macrophages. They skew macrophages towards an M2 phenotype by decreasing their production of IL-12, through IL-10.
- PMN-MDSCs may induce T cell anergy by suppressing CD3-ζ expression and inhibiting CD8+ T cells through PD-L1–PD1 interaction.
The Role of MDSCs in Cancer Progression
- MDSCs migrate to the tumor microenvironment (TME), in response to chemokines CCL5, CCL7 and CXCL8. There, they produce immunosuppressive cytokines that protect the tumor from the patient’s immune system and make the tumor resistant to immunotherapy.
- Increased circulating levels of MDSCs correlate with worse prognosis in human cancers, such as breast, colorectal, and thyroid and non-small-cell lung cancer (NSCLC). In non-solid tumors, M-MDSC numbers correlate with reduced survival in subjects with multiple myeloma, Hodgkin’s or non-Hodgkin’s lymphoma, and diffuse large B cell lymphoma.
- Recent studies demonstrated the value of MDSCs in predicting the response to various cancer therapies. The frequency of M-MDSCs is negatively correlated with the response to chemotherapy in breast, cervical, prostate and colorectal cancer, squamous cell carcinoma, multiple myeloma, and Hodgkin’s lymphoma.
- MDSCs promote angiogenesis, tumor invasion, and metastasis.
- MDSCs often differentiate into tumor associated macrophages (TAMs).
- Promote differentiation of fibroblasts to cancer-associated fibroblasts (CAFs).
Targeting MDSCs in Cancer
- MDSCs can be a therapeutic target to alleviate their pro-tumorigenic functions and immunosuppressive activities.
- Eliminating MDSCs improves the ability of the host’s immune system to attack the cancer and improves the efficacy of immunotherapy.
- Tumor-infiltrating MDSCs preferentially use fatty acid-β oxidation (FAO) as a primary source of energy. Some studies have demonstrated that inhibition of FAO affects the suppressive functions of MDSCs and enhances the efficacy of cancer immune therapy.
- MDSCs migrate to the tumor microenvironment (TME), in response to chemokines CCL5, CCL7 and CXCL8. Inhibitors of their chemokine receptors (CCR1 and CXCR2) could abrogate immune evasion and improve anti-tumor T cell responses.
Roles of MDSCs in different pathologic conditions
- Cancer was the first condition in which MDSCs were described. However, studies have demonstrated the role of these cells in the regulation of immune responses and the pathogenesis of other diseases:
- M-MDSC populations are expanded in people with different infectious diseases such as Sepsis, in Pseudomonas aeruginosa and Klebsiella pneumonia, Aspergillus fumigatus and Candida albicans infections. Recently, massive expansion of MDSCs was observed in patients with severe coronavirus disease (COVID-19) and these cells declined during the convalescent phase.
- It has been shown that MDSC populations are expanded in humans with autoimmune diseases and murine models. However, the role of MDSCs in these diseases is not established.
- The expansion of MDSC populations in obese mice and humans.
- MDSC populations are expanded in the peripheral blood and decidua during pregnancy in healthy women, and rapidly decrease to normal levels after birth.
Quiz
Now test your knowledge with these questions!
Download these Resources
References
- Tesi RJ. MDSC; the Most Important Cell You Have Never Heard Of. Trends Pharmacol Sci. 2019;40(1):4-7.
- Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19(2):108-19.
- Agrati C, Sacchi A, Bordoni V, Cimini E, Notari S, Grassi G, et al. Expansion of myeloid-derived suppressor cells in patients with severe coronavirus disease (COVID-19). Cell Death Differ. 2020;27(11):3196-207.
- Bai J, Gao Z, Li X, Dong L, Han W, Nie J. Regulation of PD-1/PD-L1 pathway and resistance to PD-1/PD-L1 blockade. Oncotarget. 2017;8(66):110693-707.
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253-68.
References
- Tesi RJ. MDSC; the Most Important Cell You Have Never Heard Of. Trends Pharmacol Sci. 2019;40(1):4-7.
- Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19(2):108-19.
- Agrati C, Sacchi A, Bordoni V, Cimini E, Notari S, Grassi G, et al. Expansion of myeloid-derived suppressor cells in patients with severe coronavirus disease (COVID-19). Cell Death Differ. 2020;27(11):3196-207.
- Bai J, Gao Z, Li X, Dong L, Han W, Nie J. Regulation of PD-1/PD-L1 pathway and resistance to PD-1/PD-L1 blockade. Oncotarget. 2017;8(66):110693-707.
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253-68.