Cancer
- Cancer treatments continue to be a major burden on health-care costs. Cancer vaccines are an effective tool for within cancer immunotherapy, designed to amplify tumor-specific T cell responses.
- Although genetic predisposition underlies some tumors and cancers, failure of immunosurveillance surrounds the disease. Cancer immunosurveillance plays an important role in the immune system, where the immune system monitoring of abnormal or transformed cells.
- Immunosurveillance can be improved by the administration of vaccines based on tumor antigens. Tumor antigens are derived from self-molecules resulting from a break in tolerance to self and therefore carry the risk of autoimmunity. However, therapeutic cancer vaccines have been extensively tested in patients with advanced cancer but have had little clinical success, which has been attributed to the immunosuppressive tumor microenvironment.
- Several mechanisms of immune suppression in cancer have been identified in tumors, including myeloid-derived suppressor cells [MDSCs], tumor-associated macrophages, other myeloid cells, and regulatory T cells. In addition, perturbation of cytokine networks, metabolism changes, the production of amino acid-degrading enzymes and indoleamine 2,3-dioxygenase 1 (IDO1), and suppressive activity of oxygen and potassium in the tumor microenvironment alter the immune response against tumor and cancer.
Cancer Vaccines
- Cancers develop from an early pre-malignant lesion, to an advanced pre-malignant lesion and malignant cancer. The pre-existing immunity can modulate the outcomes of cancer and vaccination (Figure 1).
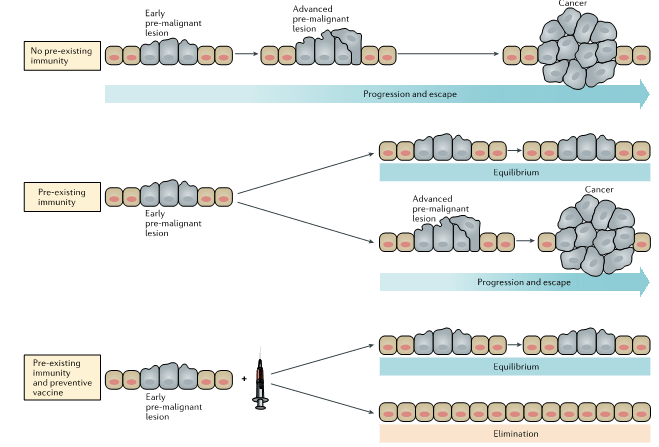
- Cancer vaccination has had only modest effects with a low rate with <7% of objective clinical responses and an overall rate of clinical benefit of around 20%.
- The presence of pre-existing T cell responses at the tumor site does not guarantee an antitumor response post-vaccination.
- Tumor antigens can be categorized as being tumor associated or tumor specific. Tumor-associated antigens (TAAs) have been characterized as targets of tumor-specific antibodies or T cells. Non-mutated and mutated antigen targets for vaccines can be identified from pre-malignant lesions. A vaccine based on mutated antigens showed immunogenicity and antitumor efficacy in animal models.
- Mutations in oncogenes and cancer suppressor genes occur very early in the process of tumorigenesis. Mutated antigens are more immunogenic than non-mutated TAAs and will elicit higher affinity antibodies and higher numbers of T cells.
- Many of TAAs are also candidates for preventive vaccines, and new antigens and antigen categories have since been defined. TAAs are excellent candidate antigens for preventive vaccines because they are proven targets of spontaneous immune surveillance.
- TAA vaccines must overcome central and acquired tolerance, limiting the magnitude of induced T cell responses.
- Cancer vaccines expressing TAAs have been developed during the last three decades, based on dendritic cells (DCs), viral or bacterial vectors, virus-like particles, ‘naked’ DNA-based, and RNA-based. However, despite applying the latest and best immunological knowledge to the design and testing of therapeutic cancer vaccines, vaccines’ overall impact on disease-free survival, and cancer recurrence has been minimal.
- Delivery systems used in cancer vaccine models have been largely suboptimal, resulting in weak and short-lived antigen-specific T cell responses.
- T cells can be profoundly affected by the long-term stay in the tumor microenvironment and exhibit an exhausted phenotype. Inhibiting the suppressors and modulating the tumor microenvironment favors the antitumor immunity.
- Checkpoint blockade immunotherapy has been showing partial help for cancer immunotherapy. The most effective immunotherapy against negative regulators of effector T cell function is monoclonal antibodies against CTLA-4, programmed cell death protein 1 (PD-1), and PD-1 ligand 1 (PD-L1).
- Studies in cancer vaccines and immunotherapy (like checkpoint inhibitors) have suggested that the combination of them can increase the efficacy of checkpoint inhibitors as well and the therapeutic efficacy of vaccines.
- Studies of pre-malignant lesions allow identification of abnormal cells at lesion sites and has an important role in immunoprevention. This can be managed by surgery for removing or screening. Vaccination at the early stages of pre-malignant lesions could boost immunosurveillance. Lesion intervention could assist in avoiding immune exhaustion (reducing the time of interaction between abnormal cells and the immune system) and contribute to a less immunosuppressive local microenvironment mediated by vaccine-elicited T cells.
- Tumor-specific antigens are foreign antigens to the body not subject to central tolerance and expressed by cancer cells. Oncogenic viral antigens have been identified in virus-induced cancers such as human papillomavirus (HPV)-associated cervical cancer and hepatitis B virus (HBV)-associated hepatocellular carcinoma.
- Vaccines against cancer-causing viruses, such as HPV and HBV, are very effective in preventing the initial infection and therefore reducing the risk of cancers caused by viruses. However, infectious etiology is unknown or nonexistent for the majority of cancers.
- Reduced frequencies of T cells against oncogenic viruses such as HPV, HBV, and Epstein–Barr virus (EBV) have been detected in patients with cancer. Various vaccines can elicit or boost T cell responses. Although the vaccines have been immunogenic and protective in animal models, the cancer vaccines showed little or no clinical efficacy in patients with advanced cervical cancer (caused by HPV), hepatocellular carcinoma (caused by HBV), and oropharyngeal carcinomas, and Hodgkin disease (caused by EBV).
- To develop successful vaccines for cancer, the following parameters must be considered: tumor antigens, antigen prioritization, antigen delivery, requirements for long-term T cell memory, and the immunocompetence of cancer patients.
- Although many unique tumor antigens, mutated neoantigens, have been identified as the predicted products of mutations revealed by exome sequencing of primary tumors, only a few tumor antigens have been confirmed as targets of spontaneous immunity and immunosurveillance.
- Tumour neoantigens are generated as products of somatic mutations. They are not only tumour specific but also highly immunogenic on the basis of lack of central tolerance.
The Human Vaccines Project
- The Human Vaccines Project is an international platform to accelerate the development of vaccines and immunotherapies against major global infectious diseases and cancers by decoding the human immune system under current technology and state of knowledge in systems biology, computational biology, structural biology, and immune system monitoring.
- The major objectives of the Human Vaccine Project are:
- Deciphering the human immunome, comprising the naïve and the adaptive repertoires in several populations, and relatedly, the human antigenome, representing the immune system targets from infected and neoplastic cells.
- Elucidating the principles of immunogenicity, such as how to elicit durable and clinically effective immune responses.
- Identifying vaccination strategies that generate and sustain effector T cell responses in large and persistent antigen burdens.
- The mutanome is the identification and description of all non-synonymous mutations in a tumor. It provides the basis for antigen discovery focusing on identifying T cell epitopes containing somatically mutated residues, referred to as mutant neoantigens.
- Studies have shown that mutant neoantigens can generate robust CD8+ T cell responses and provide efficient therapeutic vaccination.
- Next-generation sequencing (NGS) allows defining a ‘personalized’ mutanome, a promising therapeutic strategy to define the specific self-antigen epitopes and generate individualized vaccines.
- One strategy for accelerating cancer vaccine development is to study and dissect the immune fitness after vaccination in cancer patients and healthy subjects.
- Several challenges limit the development of vaccines against cancer and infectious diseases:
- A lack of understanding of the human immunome.
- Limited data on human antigenome.
- Limited understanding of optimal strategies to elicit specific and durable immune responses in humans.
- The need to identify the optimal means to harness innate immunity.
Quiz
Now test your knowledge with these questions!
Download these Resources:
References
- Finn OJ. The dawn of vaccines for cancer prevention. Nature Reviews Immunology. 2018;18:183-194.
- Finn OJ. Human tumor antigens yesterday, today, and tomorrow. Master of Immunology. Cancer Immunology Research. 2017;5:347-354.
- Romero P; et al. The Human Vaccines Project: A roadmap for cancer vaccine development. Science Translational Medicine. 2016;8:334ps9.
References
- Finn OJ. The dawn of vaccines for cancer prevention. Nature Reviews Immunology. 2018;18:183-194.
- Finn OJ. Human tumor antigens yesterday, today, and tomorrow. Master of Immunology. Cancer Immunology Research. 2017;5:347-354.
- Romero P; et al. The Human Vaccines Project: A roadmap for cancer vaccine development. Science Translational Medicine. 2016;8:334ps9.