Activation of B cells
There are two main immune responses that can drive the activation of resting B-cells, and these are termed as T-cell dependent and independent immune responses.
- Resting B cells become activated by antigen via the BCR and/or via their toll like receptors (TLR4, 7, 9 in mice) and start to proliferate.
- Protein antigens become internalized, digested and presented to T cells as peptides via MHCII.
- Cognate B cell / T cell interaction provides co-stimulation to B cells via CD40, which becomes activated on B cells via CD40 ligand (CD40L) expressed on T cells.
- T cells also provide cytokines to B cells that support their survival (IL-4), differentiation into plasma cells (IL-21, IL-6) or class switch recombination.
- In mice, Th1 cytokines, such as IFNγ, typical for antiviral responses, elicit IgG2 isotypes, Th2 cytokines, such as IL-4, IL-5 and IL-13, typical for parasite infections, elicits IgG1 and IgE responses.
- With regards to PAMPs, for instance lipopolysaccharide, they elicit differentiation of B cells into short-lived plasma cells secreting low-affinity antibodies.
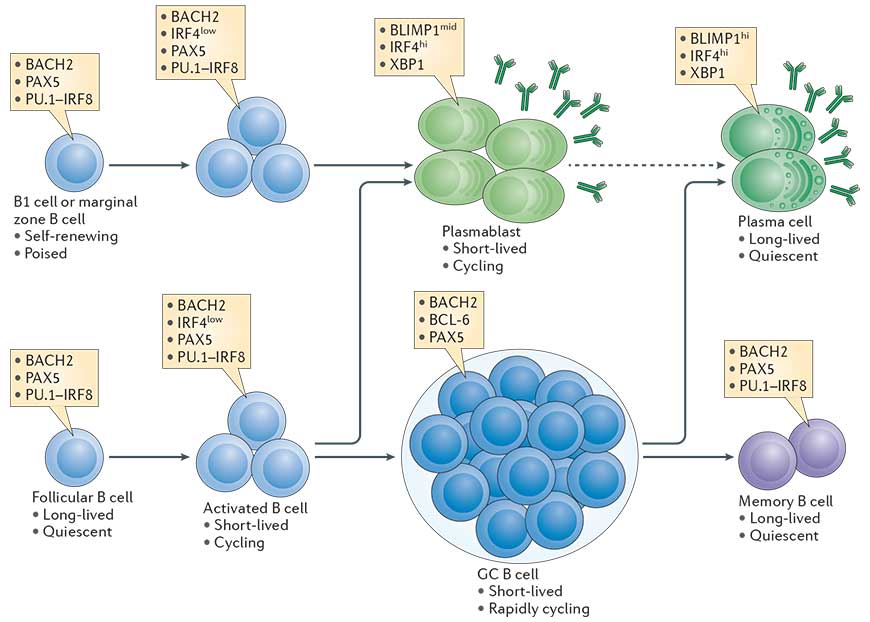
- B cell differentiation into plasma cells is coupled to a certain number of cell divisions that are required to allow expression of transcription factors which terminate the B cell program and initiate the plasma cell program. The transcriptional network that is initiated in B cells in a proliferation dependent manner and fosters plasma cell differentiation (and shows different B cell activations fates) is outlined in 1 (Taken from Nutt et al., Nature Reviews in Immunology, 2015).
- T cell dependent activation of B cells supports the generation of memory B cells, and long-lived plasma cells secreting high affinity antibodies. This process requires specific microanatomical structures in secondary lymphoid organs, the germinal centers, where class switch recombination and somatic hypermutation occur.
B cell types
- Plasma cells can be defined as terminally differentiated antibody secreting cells that fall under the B-cell lineage and are non-dividing.
- Resting B2 B cells (follicular (FO) and marginal zone (MZ) B cells) express membrane bound IgM, the B cell receptor (BCR) of the IgM type.
- B1 B cells, located primarily to the pleural cavities, are a self-renewing population with a limited repertoire. They differentiate rapidly into short lived plasma cells and secrete natural IgM in a T cell independent manner.
- MZ B cells reside in the marginal zone that surrounds the follicles of secondary lymphoid organs and is directly connected to the vasculature. MZ B cells therefore respond to blood born antigens. They react more rapidly to PAMPs and differentiate rapidly into short lived plasma cells.
- FO B cells express also membrane bound IgD, reside in the follicles of secondary lymphoid organs and are long lived. They are typically the B cells that interact with cognate T cells.
- Memory B cells express membrane bound Ig, are long lived cells and respond quickly upon reencounter of the antigen.
- ASC (antibody secreting cells) (Plasmablasts and plasma cells) are the unique population able to secrete antibodies.
The germinal center (GC) reaction
- Germinal centers (GCs) are transiently formed structures within B cell zone (follicles) in secondary lymphoid organs – ileal Peyer’s patches, and the spleen – where mature B cells are activated, proliferate, differentiate, and mutate their antibody genes. GCs play an essential part of where antibody-associated memory is generated.
- One of the fundamental pathways needed for the Germinal Center (GC) reaction is the interaction between activated follicular helper CD4 T (Tfh) cells and B cells.
- Tfh cells are essential for the GC reaction as they play a role in increasing the efficiency of antibody responses.
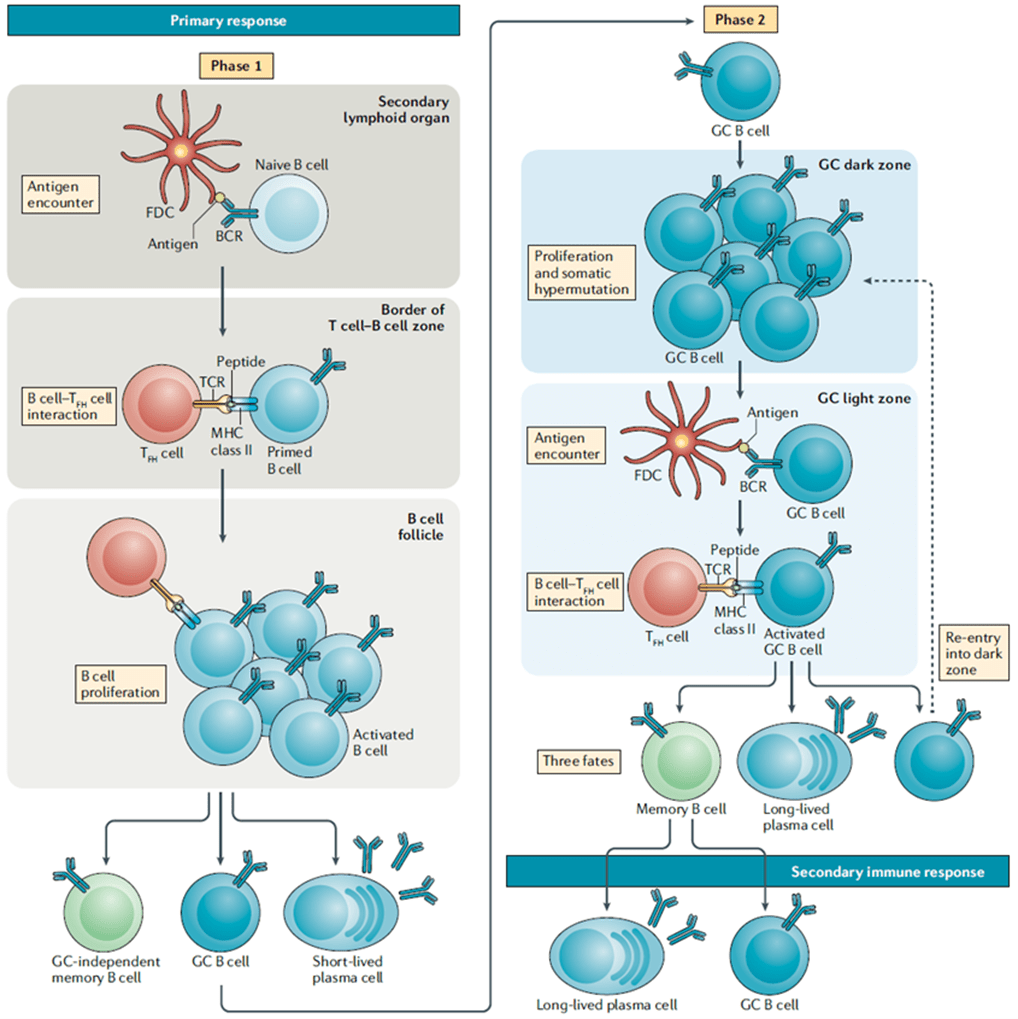
- B-cells encounter antigen in secondary lymphoid organs, thereby triggering their activation. These activated B cells will migrate to the border of the T cell zone where they will encounter T cells. (Figure 1)
- CD40 ligand on T helper cells interacts with CD40 on B cells leading to the secretion of cytokines that will encourage B-cell proliferation and isotype switching.
- The survival signals that B-cells receive from T cells allow them to continue proliferating and differentiate into short-lived plasma cells called plasmablasts or form germinal centres (GCs). (Figure 1)
- GCs are specialized regions formed within secondary lymphoid organs and are the sites taken to be responsible for generating mature B-cells, i.e. plasma cells and memory B-cells. GC formation is taken to occur in a T-cell dependant manner due to the same signals that drive Bcell proliferation also being responsible for maintenance of the GC.
- GCs are made up of light and dark zones and the sole purpose of GCs is to give rise to antibody-producing plasma cells and memory B-cells with BCRs that have a very high affinity for antigen.
- The GC reaction starts in the dark zone, where B-cells undergo rapid proliferation as well somatic hypermutation (SHM). SHM, initiated by the enzyme activation-induced cytidine deaminase (AID), will lead to random mutations in the variable regions of the BCR. Some of these mutations will result in BCRs that have a larger range of affinities for antigen, thus giving these B-cell clones higher chances of survival when they migrate to the light zone and compete for antigen with other B-cell clones.
- The light zone is taken to be the site where class switch recombination (CSR) starts, also initiated by AID, however, B-cells will migrate back and forth between both the dark and light zone as they go through multiple rounds of both SHM and CSR.
Antibody secreting cells (ASC)
- Early ASC still proliferate and are also called plasmablasts (PB) or short-lived plasma cells. Extra-follicular PB are generated in a Germinal-center independent manner and are located outside the B follicle. These EFPB could produce pathogen specific antibodies, mostly IgM but also of different isotypes (class switched)
- Plasma cells (PC) or long-lived plasma cells, predominantly derived from GC B cells, are terminally differentiated, non-proliferating plasma cell that secretes high amounts of antibody. PC are much larger than resting or even proliferating B cells.
- The development of plasma cells from antigen-activated B cells is accompanied by a fundamental change in their transcriptional profile and the transition from a proliferating (i.e., plasmablasts) to a non-dividing stage (i.e., plasma cells).
- In plasma cells and plasmablasts, the exons coding for the membrane domain of Ig molecules of various isotypes have been removed and replaced by exons coding for sequences allowing antibody secretion.
- ASC have a strongly expanded endoplasmic reticulum and express high amounts of molecules involved in antibody folding and secretion and can secrete several thousands of antibodies per second.
- Long-lived plasma cells migrate back to the bone marrow where their survival is supported in niches. Stable antibody titers upon vaccination have been observed for 20 years and more for some immunogens.
Immunological Memory
Immunological memory can be thought of as the ability of immune cells to interact with a pathogen or microorganism thereby leading to a change in the way the immune system responds to this same pathogen the second time that it encounters it.
With regards to memory, B-cells are taken to contribute to this in two ways, that is, through the development of plasma cells as well as memory B-cells.
Why are memory responses better than primary responses?
- Altered migration pattern: are poised ready at the site of infection
- Higher affinity: more sensitive, respond to lower levels of infection
- Enhanced effector response
Memory B cells
- Memory B cells are also long-lived, are formed after a primary response and are taken to be dormant until they have a secondary encounter to an infection. These cells are known to give quite a rapid and almost immediate response to re-infection.
- Memory B cells (MBC) are persistent antigen-specific cells that mediate rapid and vigorous antibody responses during secondary immune responses.
- MBC are generated from naïve pre-immune progenitors during immunization or infection and are heterogeneous in phenotype and function.
- MBC differentiate from activated B cells and Germinal Center B cells.
- To Identify this population, MBC must be distinguished from activated B cells, GC B cells and Plasmablast/plasma cells.
- MBC frequently express class-switched immunoglobulin isotypes, and as a population their affinity for antigen is higher than among their naïve precursors. (Most of MBC are somatically mutated and class switched), but memory B-cells do not go through a selection process that is as highly and tightly regulated as is the case for Plasma cells. This provides an advantage for memory B-cells in that they get to maintain a broader range of affinities and specificities to antigens
- Neither immunoglobulin class-switch recombination (CSR) nor SHM are necessarily required for MBC formation, MBCs can be either IgM+ or class-switched and can be either unmutated or mutated.
- The spleen is the major reservoir of MBC in humans and mice. They also can be found in others secondary lymph organs and in blood.
- MBC vary markedly in their intrinsic functional properties: they can rapidly differentiate into antibody-secreting plasma cells or seed of secondary germinal centers.
- PD-L2, CD80, and CD73 expression define memory B cell subsets in mice.
Roles of long- lived plasma cells and memory B cells in protective immunity
- Current models of B cell memory provide a framework that suggests that long- lived plasma cells in the bone marrow that were highly selected for, and that produce high-affinity antibodies, form the first line of defence against disease-causing pathogens, whereas memory B cells come through to provide secondary defence in the event that pathogens escape long-lived plasma cell- mediated protection.
- Memory B-cells can be activated by pathogens to differentiate into long- lived plasma cells or re-enter the GCs so as to replenish the memory B cell pool.
The generation of antibody diversity
- Antibodies (Abs) have different functions: neutralizing antigens, triggering effector mechanisms such as phagocytosis, complement, antibody – dependent cellular cytotoxicity, opsonizing, promoting acute inflammatory responses (activating mast cells, recruiting macrophages), regulating B cell response (and other cell populations), among others.
- Abs are Y – or T – shaped molecules in which the arms of the molecule (Fab) recognize foreign material and the stem (Fc) interacts with immune molecules that lead to the elimination of the foreign material.
- The antibody basic unit consists of two identical heavy and two identical light chains held together by interchain disulfide bonds. The light chains exist in two forms known as kappa (κ) and lambda (λ). The heavy chains (γ, μ, α, δ and ε) determine the antibody isotype and its function.
- The N – terminal parts of the heavy chains and the light chains form the two identical Fab arms that are linked to the Fc stem of the molecule consisting of the C – terminal parts of the heavy chains.
- The extremities of the Fab arms consist of regions of variable amino acid sequences that are involved in binding antigen and thereby give each antibody its unique specificity.
- Complementarity determining regions (CDRs) are hypervariable regions associated with antigen recognition, located in the amino – terminal regions of heavy and light chains, (in Fab region)
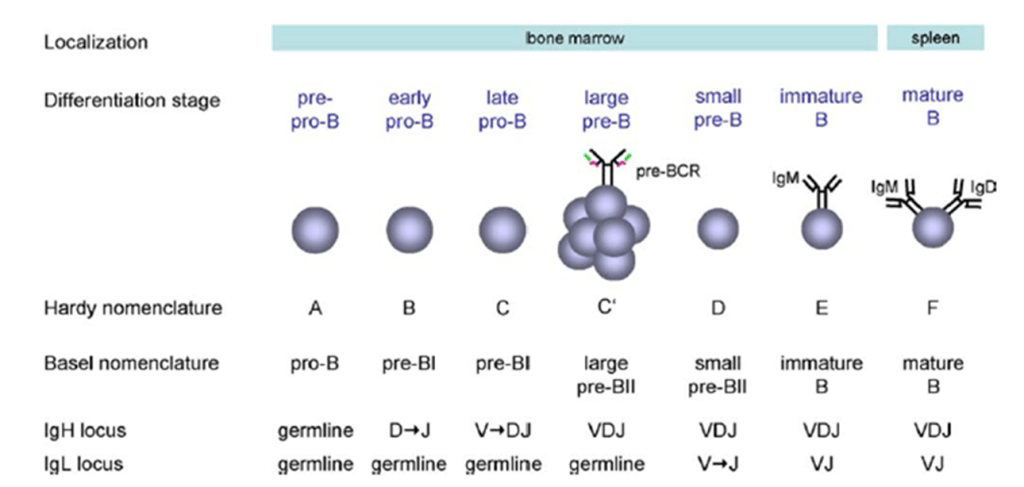
- The antibody repertoire of an individual is generated through somatic recombination events from a limited set of germline gene segments, a process called V(D)J recombination that occurs without antigen presence. Figure 2 (C. Vettermann et al. / Seminars in Immunology 18 (2006) 44–55).
- The variable light and heavy chain loci in humans contain multiple gene segments, which are joined to produce the final V region exon
- The human heavy chain variable region is generated by joining of VH, D and J gene segments and the light chain variable regions (κ and λ) by joining of VL and J segments.
- After somatic recombination, the rearranged DNA is transcribed, the RNA transcript is spliced to bring together the V region exon and the C – region exon, and lastly the spliced mRNA is translated to produce the final immunoglobulin protein.
- Class switch recombination (CSR) events allow the same antibody specificity (variable regions) to be associated with different antibody classes and subclasses (constant regions) and therefore with different functions. CSR occurs after B cell activation and with the action of AID.
- Somatic hypermutation, which occurs following antigen activation involves the introduction of point mutations into V regions of rapidly proliferating B – cells in the germinal centers of Lymphoid follicles. The variable regions of immunoglobulin heavy and light chains are further diversified by this mechanism.
- The enzyme activation – induced cytidine deaminase (AID) has been demonstrated to be essential for both somatic hypermutation and class – switch recombination.
Quiz
Download these Resources:
Related Talk
Richard Koup, NIH – Germinal centres and tfh cells
References
- Akkaya, M., Kwak, K., & Pierce, S. K. (2019). B cell memory: building two walls of protection against pathogens. Nature Reviews Immunology, 20(4), 229–238. https://doi.org/10.1038/s41577-019-0244-2
- Cancro, M. P., & Tomayko, M. M. (2021). Memory B cells and plasma cells: The differentiative continuum of humoral immunity. Immunological reviews, 303(1), 72–82. https://doi.org/10.1111/imr.13016
- Cooper M. D. (2015). The early history of B cells. Nature reviews. Immunology, 15(3), 191–197. https://doi.org/10.1038/nri3801
- Peter J. Delves, Seamus J. Martin, Dennis R. Burton, Ivan M. Roitt. Roitt’s Essential Immunology, Twelfth Edition. Chapter 3. Antibodies.
- Schuh, W., Mielenz, D., & Jäck, H. M. (2020). Unraveling the mysteries of plasma cells. Advances in immunology, 146, 57–107. https://doi.org/10.1016/bs.ai.2020.01.002
- Vettermann, C., Herrmann, K., & Jäck, H. M. (2006). Powered by pairing: the surrogate light chain amplifies immunoglobulin heavy chain signaling and pre-selects the antibody repertoire. Seminars in immunology, 18(1), 44–55. https://doi.org/10.1016/j.smim.2006.01.001
- Weisel, F., & Shlomchik, M. (2017). Memory B Cells of Mice and Humans. Annual review of immunology, 35, 255–284. https://doi.org/10.1146/annurev-immunol-041015-055531
References
- Akkaya, M., Kwak, K., & Pierce, S. K. (2019). B cell memory: building two walls of protection against pathogens. Nature Reviews Immunology, 20(4), 229–238. https://doi.org/10.1038/s41577-019-0244-2
- Cancro, M. P., & Tomayko, M. M. (2021). Memory B cells and plasma cells: The differentiative continuum of humoral immunity. Immunological reviews, 303(1), 72–82. https://doi.org/10.1111/imr.13016
- Cooper M. D. (2015). The early history of B cells. Nature reviews. Immunology, 15(3), 191–197. https://doi.org/10.1038/nri3801
- Peter J. Delves, Seamus J. Martin, Dennis R. Burton, Ivan M. Roitt. Roitt’s Essential Immunology, Twelfth Edition. Chapter 3. Antibodies.
- Schuh, W., Mielenz, D., & Jäck, H. M. (2020). Unraveling the mysteries of plasma cells. Advances in immunology, 146, 57–107. https://doi.org/10.1016/bs.ai.2020.01.002
- Vettermann, C., Herrmann, K., & Jäck, H. M. (2006). Powered by pairing: the surrogate light chain amplifies immunoglobulin heavy chain signaling and pre-selects the antibody repertoire. Seminars in immunology, 18(1), 44–55. https://doi.org/10.1016/j.smim.2006.01.001
- Weisel, F., & Shlomchik, M. (2017). Memory B Cells of Mice and Humans. Annual review of immunology, 35, 255–284. https://doi.org/10.1146/annurev-immunol-041015-055531