What are tumor-infiltrating lymphocytes (TIL)?
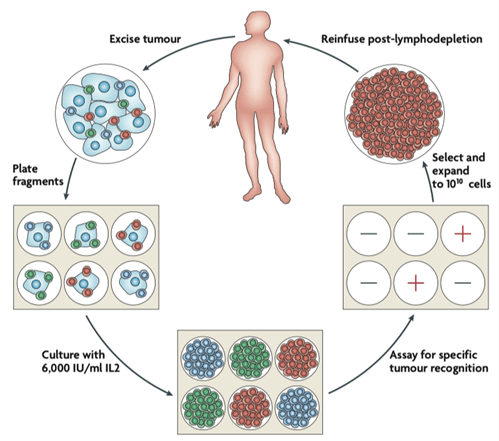
- Tumor infiltrating lymphocytes (TILs) are lymphocyte populations that have migrated to and infiltrate tumors. TILs recognise tumor specific antigens and elicit direct and indirect cytotoxic activity that can lead to a reduction in tumor burden or even cure in some patients. TIL therapy, a type of adoptive cell therapy (ACT), consists of the extraction of T cells from a patient’s tumor, the ex vivo expansion of TILs, and the reinfusion of the expanded T cells into the patient.
- Both CD8+ T cells and CD4+ T cells may be necessary to mediate tumor rejection.
- CD8+ TIL may have a different co-inhibitory and co-stimulatory expression patterns than CD4 T cells, and express molecules such as: PD-1, LAG3, TIM3, and 4-1BB and contribute to cytolytic activity when stimulated with autologous tumor cells.
- The therapy dates as back as 1986 when Steve Rosenberg, Chief of the Surgery Branch of the National Institutes of Health, treated metastatic melanoma patients with TIL.
How are TIL produced?
- Surgically-resected tumors (usually melanoma, as they consistently contain TILs with antitumor reactivity) are cultured ex vivo in medium
- Lymphocytes from these cultures are tested for reactivity against tumors in co-culture assays or with a panel of human leukocyte antigen (HLA) matched allogeneic melanoma cell lines. Only reactive lymphocytes undergo further culture.
- Culture medium usually contains growth factors such as IL-2 and CD3 agonists to stimulate T cell proliferation and facilitate T cell expansion, which requires five to six weeks to produce enough cells (around 50 billions) to be infused into the patient.
- Through more advanced techniques, such as tumor DNA/RNA isolation and whole exome or transcriptome sequencing, tumor neoantigen-reactive T cells are selected and expanded, although this is in early stages right now. (This selection can lead to longer production steps: more time, a resource cancer patients may lack).
Is any treatment required prior to TIL infusion?
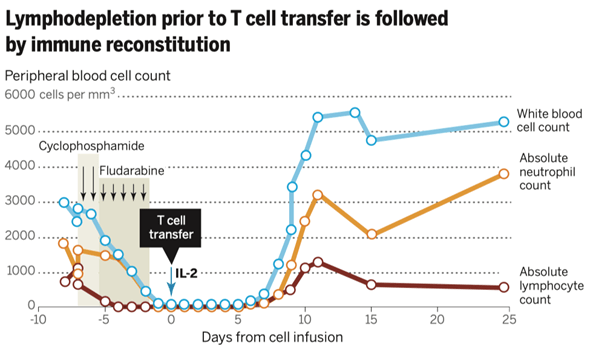
- Patients usually undergo chemotherapy (with drugs such as cyclophosphamide and fludarabine) or total body irradiation to deplete lymphocytes (lymphodepletion) previous to TIL infusion.
- This step may be necessary, as some regulatory T cells (CD4+ FoxP3+) and other lymphocytes may compete with TIL for homeostatic molecules such as IL7 and IL-15 (a T cell growth factor).
- Following TIL therapy, immune reconstitution by remaining peripheral lymphocytes is observed (a process called homeostatic expansion).
- IL-15 and IL-7 levels increase after lymphodepletion.
- Infused syngeneic lymphocytes are more likely to expand and engraft in vivo.
How can TIL therapy be enhanced?
- Lymphodepletion prior to infusion.
- Proper characterization and selection of cells prior to infusion.
- T cells with a CCR7+, CD27+, CD28+, CD62L+ phenotype (also known as central memory cells, a less differentiated phenotype) have more effective anti-tumour activity than highly differentiated cells.
- TIL with longer telomeres have shown greater response.
- Additionally, intravenous IL-2 is used as a T cell growth factor, usually following TIL infusion.
- Host immunization with a tumour antigen vaccine.
- Injection of Toll-like receptor agonists.
- Anti-tumour TCR encoding retrovirus transduction.
What kind of tumors could benefit from TIL therapy?
- Melanoma and cervical cancer are the ones with most evidence. This has been attributed to their high immunogenicity.
Table 1. Selected clinical trials of TIL and related adoptive cell therapy
[table id=241 /]
Table 1. Selected clinical trials of TIL and related adoptive cell therapy [Nature Biotechnology 37, 969-971 (2019)]
- TIL therapy may be used when patients are resistant to first and second line therapies (such as chemotherapy, check point inhibitor therapy).
- In different trials, up to 20% of patients have had complete regression with no recurrence in 5-10 years follow up.
- Ovarian, kidney (renal cell carcinoma), gastrointestinal (adenocarcinomas), and head and neck cancers (squamous cell carcinoma) have indirect evidence of tumor infiltrating T cells presence/density considered as a good prognostic factor. Direct evidence of TIL therapy in these cancers is scarce.
TIL therapy pitfalls
- TIL is a patient-specific therapy that requires laboratory expertise and is both labour and resource-intensive
- Initially (before lymphodepletion was used) transferred cells had short in vivo persistence
- Side effects of associated therapy: chemotherapy (such as febrile neutropenia), IL-2 (capillary leak syndrome: fever, oedema, shortness of breath)
Quiz
Download these Resources:
References
- Nature Biotechnology 37, 969-971 (2019)
- Nature Reviews Cancer 8, 299–308(2008)
- Science 348 (6230), 62-68
- Molecular Oncology 9 (2015) 1918 – 1935
- Journal for ImmunoTherapy of Cancer (2018) 6:102
References
- Nature Biotechnology 37, 969-971 (2019)
- Nature Reviews Cancer 8, 299–308(2008)
- Science 348 (6230), 62-68
- Molecular Oncology 9 (2015) 1918 – 1935
- Journal for ImmunoTherapy of Cancer (2018) 6:102