Candidate gene studies
- Due to the different outcomes of an TB infection and the fact that most infected individuals remain asymptomatic, several studies focused on the possibility that host genetic factors may play a role in resistance and susceptibility to tuberculosis
- Researchers became aware of this possibility because of the fact that close relatives of TB cases were at higher risk to develop TB than unrelated persons who also came in close contact with the patient [1]
- Studies with twins followed and found that the accordance of TB among monozygotic twins was significantly higher than between dizygotic twins
- The heritability of reacting to tuberculosis infection was also shown in healthy twins through TSTs and IGRAs: the heritability of TST as a categorical trait was estimated to be 71% in the Gambia, whereas the reactivity of IGRA was 39% [6]; in Chile, the heritability of TST reactivity was even higher with an estimated 92% in TB exposed children [7]
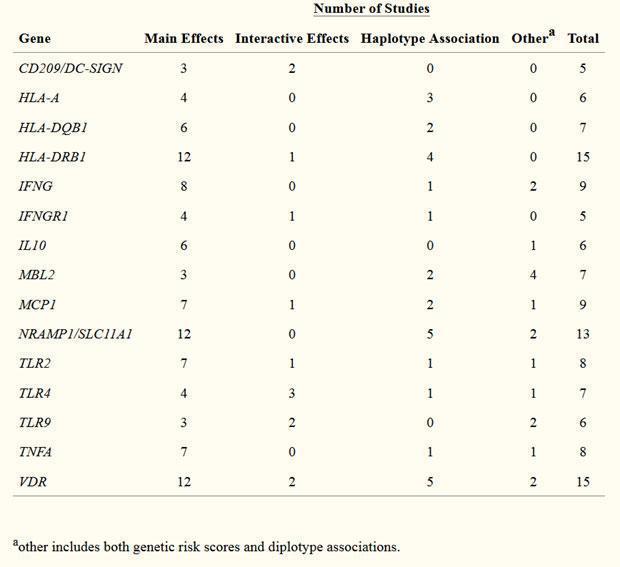
- Several candidate gene studies have been conducted to identify host genetic factors involved in TB risk; the most convincing findings, significantly associated with TB risk are genetic variants of the following genes [8]: NRAMP1, TNF, vitamin D receptor, IFNɣ, IFNɣ receptor 1, IL10, TLR2, TLR4, TLR 9 and several HLA-DRB1 haplotypes (Table 1)
- Unfortunately, results of independent studies concerning genetic associations are poorly consistent and replicative which could be due to different study designs, population genetic differences and underpowered studies
Resistance and susceptibility to mycobacteria
- The idea of the gene variants possibly playing a role in genetic susceptibility (to mycobacterial infection and disease) came from Mendel
- Severe tuberculosis in children with primary immunodeficiencies gave the hint that TB during childhood might follow Mendelian predisposition [9]
- A rare immunodeficiency syndrome is Mendelian Susceptibility to Mycobacterial Diseases (MSMD) which is characterized by a restricted vulnerability to poorly virulent, non-tuberculous mycobacteria such as BCG vaccines and environmental mycobacteria [10], like M.avium, but also other infections have been reported, like Salmonella spp. and Listeria monocytogenes.
- Studies revealed that MSMD is primarily associated with defects in the IFNɣ pathway, more precisely with mutations in the genes of some of the components of this pathway [9]: IFNɣR 1 and 2, STAT1, IRF8, CYBB, IL12β, IL12Rβ1, NEMO and ISG15
- The most common genetic defect is complete IL12Rβ1 deficiency in patients with severe tuberculosis [11], leading to insufficient IFNɣ production and thereby diminishing its function: affecting activity of macrophages and dendritic cells for anti-mycobacterial defense and antigen processing
- Those patients can be treated with human recombinant IFNɣ
- Components of other signalling pathways play also a role in susceptibility to TB; especially in infection with M.bovis, which causes TB in cattle but is also infectious in human beings, it was shown that SNPs (single nucleotide polymorphisms) in TLR2 were associated with susceptibility to this bacterium in cattle [12]
- Increased susceptibility to M.avium was seen in cattle with SNPs in TLR2 and TLR4 [13]
Other risk factors for impairment of host resistance to M. tuberculosis and anti-TB drugs
- As already mentioned, immunodeficiencies in individuals have an important impact on the resistance to Mycobacteria
- Not only classic primary immunodeficiencies, like chronic granulomatous disease or complete TYK2 deficiency, but also acquired immunodeficiency, such as HIV infection or anti-TNF treatment might favour impairment of resistance to tuberculosis [14]
- It is known that of globally 8 new TB cases 1 is HIV positive and 1 in 4 HIV-infected people die due to TB [15]; it is also important to note that a HIV infection leads to a more rapid progression from latent to active TB in a TB infected person
- Age as a factor is also considered as a study in populations from Morocco and Madagascar found an age-dependent association between TB and variants of the TOX gene
- TOX encodes a protein that is highly expressed in the thymus and is involved in the development of T lymphocytes and the study showed that variants of this gene were strongly associated with the development of TB onset before the age of 25 years [16]
- Medical treatment of TB is partly performed with isoniazid together with rifampicin, pyrazinamide and streptomycin – antibiotics that are used to treat several types of bacterial infections
- For multidrug-resistant tuberculosis, linezolid is the drug of choice, which is supposed to inhibit the mitochondrial protein synthesis [17]
Quiz
Download these Resources:
References
- Stein, C.M., et al., Genomics of human pulmonary tuberculosis: from genes to pathways. Curr Genet Med Rep, 2017. 5(4): p. 149-166.
- https://www.who.int/tb/publications/global_report/tb18_ExecSum_web_4Oct18.pdf?ua=1, W., World Tuberculosis Report. 2018.
- Moller, M., et al., Genetic Resistance to Mycobacterium tuberculosis Infection and Disease. Front Immunol, 2018. 9: p. 2219.
- Mathema, B., et al., Molecular epidemiology of tuberculosis: current insights. Clin Microbiol Rev, 2006. 19(4): p. 658-85.
- Nayak, S. and B. Acharjya, Mantoux test and its interpretation. Indian Dermatol Online J, 2012. 3(1): p. 2-6.
- Jepson, A., et al., Genetic regulation of acquired immune responses to antigens of Mycobacterium tuberculosis: a study of twins in West Africa. Infect Immun, 2001. 69(6): p. 3989-94.
- Sepulveda, R.L., et al., Tuberculin reactivity after newborn BCG immunization in mono- and dizygotic twins. Tuber Lung Dis, 1994. 75(2): p. 138-43.
- Azad, A.K., W. Sadee, and L.S. Schlesinger, Innate immune gene polymorphisms in tuberculosis. Infect Immun, 2012. 80(10): p. 3343-59.
- Boisson-Dupuis, S., et al., Inherited and acquired immunodeficiencies underlying tuberculosis in childhood. Immunol Rev, 2015. 264(1): p. 103-20.
- Bustamante, J., et al., Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Semin Immunol, 2014. 26(6): p. 454-70.
- de Beaucoudrey, L., et al., Revisiting human IL-12Rbeta1 deficiency: a survey of 141 patients from 30 countries. Medicine (Baltimore), 2010. 89(6): p. 381-402.
- Bhaladhare, A., et al., Single nucleotide polymorphisms in toll-like receptor genes and case-control association studies with bovine tuberculosis. Vet World, 2016. 9(5): p. 458-64.
- Mucha, R., et al., Toll-like receptors TLR1, TLR2 and TLR4 gene mutations and natural resistance to Mycobacterium avium subsp. paratuberculosis infection in cattle. Vet Immunol Immunopathol, 2009. 128(4): p. 381-8.
- Abel, L., et al., Genetics of human susceptibility to active and latent tuberculosis: present knowledge and future perspectives. Lancet Infect Dis, 2018. 18(3): p. e64-e75.
- Dye, C., et al., Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA, 1999. 282(7): p. 677-86.
- Grant, A.V., et al., Age-dependent association between pulmonary tuberculosis and common TOX variants in the 8q12-13 linkage region. Am J Hum Genet, 2013. 92(3): p. 407-14.
- Soriano, A., O. Miro, and J. Mensa, Mitochondrial toxicity associated with linezolid. N Engl J Med, 2005. 353(21): p. 2305-6.
References
- Stein, C.M., et al., Genomics of human pulmonary tuberculosis: from genes to pathways. Curr Genet Med Rep, 2017. 5(4): p. 149-166.
- https://www.who.int/tb/publications/global_report/tb18_ExecSum_web_4Oct18.pdf?ua=1, W., World Tuberculosis Report. 2018.
- Moller, M., et al., Genetic Resistance to Mycobacterium tuberculosis Infection and Disease. Front Immunol, 2018. 9: p. 2219.
- Mathema, B., et al., Molecular epidemiology of tuberculosis: current insights. Clin Microbiol Rev, 2006. 19(4): p. 658-85.
- Nayak, S. and B. Acharjya, Mantoux test and its interpretation. Indian Dermatol Online J, 2012. 3(1): p. 2-6.
- Jepson, A., et al., Genetic regulation of acquired immune responses to antigens of Mycobacterium tuberculosis: a study of twins in West Africa. Infect Immun, 2001. 69(6): p. 3989-94.
- Sepulveda, R.L., et al., Tuberculin reactivity after newborn BCG immunization in mono- and dizygotic twins. Tuber Lung Dis, 1994. 75(2): p. 138-43.
- Azad, A.K., W. Sadee, and L.S. Schlesinger, Innate immune gene polymorphisms in tuberculosis. Infect Immun, 2012. 80(10): p. 3343-59.
- Boisson-Dupuis, S., et al., Inherited and acquired immunodeficiencies underlying tuberculosis in childhood. Immunol Rev, 2015. 264(1): p. 103-20.
- Bustamante, J., et al., Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Semin Immunol, 2014. 26(6): p. 454-70.
- de Beaucoudrey, L., et al., Revisiting human IL-12Rbeta1 deficiency: a survey of 141 patients from 30 countries. Medicine (Baltimore), 2010. 89(6): p. 381-402.
- Bhaladhare, A., et al., Single nucleotide polymorphisms in toll-like receptor genes and case-control association studies with bovine tuberculosis. Vet World, 2016. 9(5): p. 458-64.
- Mucha, R., et al., Toll-like receptors TLR1, TLR2 and TLR4 gene mutations and natural resistance to Mycobacterium avium subsp. paratuberculosis infection in cattle. Vet Immunol Immunopathol, 2009. 128(4): p. 381-8.
- Abel, L., et al., Genetics of human susceptibility to active and latent tuberculosis: present knowledge and future perspectives. Lancet Infect Dis, 2018. 18(3): p. e64-e75.
- Dye, C., et al., Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA, 1999. 282(7): p. 677-86.
- Grant, A.V., et al., Age-dependent association between pulmonary tuberculosis and common TOX variants in the 8q12-13 linkage region. Am J Hum Genet, 2013. 92(3): p. 407-14.
- Soriano, A., O. Miro, and J. Mensa, Mitochondrial toxicity associated with linezolid. N Engl J Med, 2005. 353(21): p. 2305-6.