Immunity to Ebola
Ebola Virus
- Ebola virus (EBOV) is a negative-sense ssRNA virus belonging to the family Filoviridae of the order Mononegavirales.
- The genus EBOV includes five known virus species, which are named by the region where they were originally identified: Bundibugyo ebolavirus, Reston ebolavirus, Sudan ebolavirus, Taï Forest ebolavirus (former Côte d’Ivoire ebolavirus), and Zaire ebolavirus.
- EBOV had first been described after outbreaks of Ebola virus Disease (EVD) in southern Sudan and Zaire in 1976.
Ebola Pathology
- Ebola virus disease (EVD) is a complex syndrome and the host response is as critical in defining the disease outcome as the virus. Host genetic background probably influences the spectrum of clinical EVD manifestations with differential host responses in a large and genetically diverse human population.
- EVD can have diverse symptoms, like gastrointestinal symptoms or in severe cases Ebola hemorrhagic fever (EHF) and generally high case fatality rates.
- Infection occurs through breaks in the skin or mucosal surfaces after exposure to infected body fluids, or through parenteral transmission. First APCs of the myeloid lineage (macrophages and DCs) are infected. This is followed by triggering of inflammatory processes, leading to recruitment of additional susceptible APCs. This generates a massive pro-inflammatory response causing the systemic immunopathology seen in EVD.
- Infected macrophages and DCs then migrate to lymphoid organs, infecting more cells thereby amplifying virus production. Infected APCs can induce apoptosis in Lymphocytes thus impairing an effective immune response.
- Subsequently liver cells (hepatocytes and Kupffer cells) are infected, causing progressive acute hepatitis.
- In the late events of infection, endothelial cells lining blood vessels are infected and thereby activated, resulting in increased permeability of blood vessels and leakage, bleeding, and impaired coagulation. This is further aided by high levels of circulating inflammatory cytokines and chemokines.
Ebola Infection
Attachment and Entry
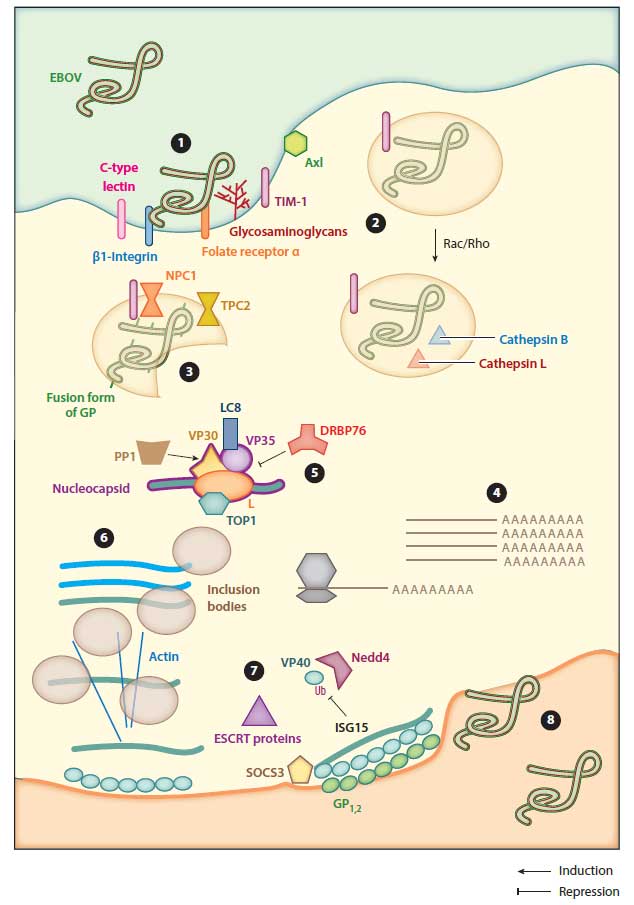
- EBOV virions bind to various cell surface molecules (e.g. C-type lectins) and enter the cell by micropinocytosis, due to the large filovirus particles. Maturation and acidification of vesicles into late endosomes triggers proteolytic processing of glycoprotein (GP), generating fusion-ready forms of GP. Fusion is then followed by release of the genome into the cytoplasm.
Transcription, Protein Synthesis, and Genome Replication
- Transcription of viral genes occurs in the cytoplasm of the host cell. First, monocistronic and polyadenylated viral gene mRNA is transcribed from the negative-strand genome. Transcription is activated by VP30, a component of the nucleocapsid complex.
Virion Assembly and Release
- Virus assembly starts by bringing nucleocapsid proteins into proximity with newly synthesized genomes. Complete assembled virions then leave the cell by budding through the plasma membrane.
Immune evasion mechanisms of Ebola
- EBOV infects DC and MO and kills lymphocytes, thereby preventing antigen presentation and T cell effector functions.
- The minor matrix protein VP24 inhibits IFN signalling by reducing host cell sensitivity to IFNs and inhibiting expression of interferon stimulated genes. VP24 further modulates MAPK activation and blocks DC maturation.
- VP35 also antagonizes IFN signalling and further hides dsRNA preventing virus sensing which would be followed by a shutdown of viral protein translation.
- EBOV further produces a soluble form of GP (sGP) catching and neutralizing antibodies against GP on the surface of virions.